Outcome reporting in neonates experiencing withdrawal following opioid exposure in pregnancy: a systematic review
- PMID: 32164782
- PMCID: PMC7069160
- DOI: 10.1186/s13063-020-4183-9
Outcome reporting in neonates experiencing withdrawal following opioid exposure in pregnancy: a systematic review
Abstract
Background: Neonatal withdrawal secondary to in utero opioid exposure is a growing global concern stressing the psychosocial well-being of affected families and scarce hospital resources. In the ongoing search for the most effective treatment, randomized controlled trials are indispensable. Consistent outcome selection and measurement across randomized controlled trials enables synthesis of results, fostering the translation of research into practice. Currently, there is no core outcome set to standardize outcome selection, definition and reporting. This study identifies the outcomes currently reported in the literature for neonates experiencing withdrawal following opioid exposure during pregnancy.
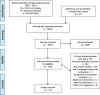
Methods: A comprehensive literature search of MEDLINE, EMBASE and Cochrane Central was conducted to identify all primary research studies (randomized controlled trials, clinical trials, case-controlled studies, uncontrolled trials, observational cohort studies, clinical practice guidelines and case reports) reporting outcomes for interventions used to manage neonatal abstinence syndrome between July 2007 and July 2017. All "primary" and "secondary" neonatal outcomes were extracted by two independent reviewers and were assigned to one of OMERACT's core areas of "pathophysiological manifestation", "life impact", "resource use", "adverse events", or "death".
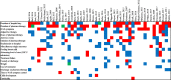
Results: Forty-seven primary research articles reporting 107 "primary" and 127 "secondary" outcomes were included. The most frequently reported outcomes were "duration of pharmacotherapy" (68% of studies, N = 32), "duration of hospital stay" (66% of studies, N = 31) and "withdrawal symptoms" (51% of studies, N = 24). The discrepancy between the number of times an outcome was reported and the number of articles was secondary to the use of composite outcomes. Frequently reported outcomes had heterogeneous definitions or were not defined by the study and were measured at different times. Outcomes reported in the literature to date were mainly assigned to the core areas "pathophysiologic manifestations" or "resource use". No articles reported included parent or former patient involvement in outcome selections.
Conclusions: Inconsistent selection and definition of primary and secondary outcomes exists in the present literature of pharmacologic and nonpharmacologic interventions for managing opioid withdrawal in neonates. No studies involved parents in the process of outcome selection. These findings hinder evidence synthesis to generate clinically meaningful practice guidelines. The development of a specific core outcome set is imperative.
Keywords: Core outcome set; Maternal opioid use disorder; Neonatal abstinence syndrome; Neonatal opioid withdrawal syndrome; Neonatal withdrawal syndrome; Opioid exposed newborn baby.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Canadian Paediatric Society. Managing infants born to mothers who have used opioids during pregnancy. Available at: https://www.cps.ca/en/documents/position/opioids-during-pregnancy. Accessed 8 July 2018. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

