Toll-like Receptors and the Control of Immunity
- PMID: 32164908
- PMCID: PMC9358771
- DOI: 10.1016/j.cell.2020.02.041
Toll-like Receptors and the Control of Immunity
Abstract
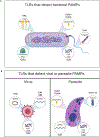
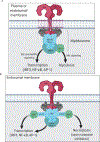
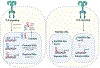
The study of innate immunity and its link to inflammation and host defense encompasses diverse areas of biology, ranging from genetics and biophysics to signal transduction and physiology. Central to our understanding of these events are the Toll-like receptors (TLRs), an evolutionarily ancient family of pattern recognition receptors. Herein, we describe the mechanisms and consequences of TLR-mediated signal transduction with a focus on themes identified in the TLR pathways that also explain the operation of other immune signaling pathways. These themes include the detection of conserved microbial structures to identify infectious agents and the use of supramolecular organizing centers (SMOCs) as signaling organelles that ensure digital cellular responses. Further themes include mechanisms of inducible gene expression, the coordination of gene regulation and metabolism, and the influence of these activities on adaptive immunity. Studies in these areas have informed the development of next-generation therapeutics, thus ensuring a bright future for research in this area.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


References
-
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, and Miyake K. (2000). Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol 164, 3471–3475. - PubMed
-
- Akira S, and Hoshino K. (2003). Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J Infect Dis 187 Suppl 2, S356–363. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, and Flavell RA (2001). Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738. - PubMed
-
- Anders HJ (2005). A Toll for lupus. Lupus 14, 417–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

