Management of dermatologic adverse events from cancer therapies: recommendations of an expert panel
- PMID: 32165025
- PMCID: PMC7175407
- DOI: 10.1016/j.abd.2020.01.001
Management of dermatologic adverse events from cancer therapies: recommendations of an expert panel
Abstract
With the development of new cancer therapies, systemic toxicity profile and effects on survival achieved an important improvement. However, a constellation of toxicities has emerged, even more remarkably, cutaneous adverse events. This report, developed by a board of Brazilian experts in oncodermatology, aims to establish a guideline for the dermatological care of oncologic patients. When possible, evidence-based recommendations were made, but in many cases, when strong evidence was not available, a consensus was reached, based on some data supporting therapies combined with personal experiences.
Keywords: Antineoplastic agents; Antineoplastic agents, immunological; Dermatology; Drug-related side effects and adverse reactions; Medical oncology; Molecular targeted therapy.
Copyright © 2020 Sociedade Brasileira de Dermatologia. Published by Elsevier España, S.L.U. All rights reserved.
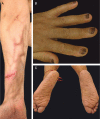
Figures






References
-
- Balagula Y., Rosen S.T., Lacouture M.E. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624–635. - PubMed
-
- Reyes-Habito C.M., Roh E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203. - PubMed
-
- Reyes-Habito C.M., Roh E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

