Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer
- PMID: 32165095
- PMCID: PMC7689684
- DOI: 10.1016/j.euo.2020.02.003
Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer
Abstract
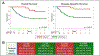
Cisplatin-based neoadjuvant chemotherapy (NAC) has demonstrated an overall survival (OS) benefit in muscle-invasive bladder cancer (MIBC). However, only a subset of patients (25-50%) have a pathologic complete response at cystectomy. Using a cohort of 58 patients from two phase 2 trials, our group previously reported that mutations in the ATM, RB1, and FANCC genes correlate with complete response to cisplatin-based NAC, and consequently improve OS and disease-specific survival (DSS). These trials enrolled patients with T2-4 (N0 or N1) MIBC and treated them with accelerated/dose-dense NAC with methotrexate, vinblastine, adriamycin, and cisplatin, or gemcitabine and cisplatin, with a plan for curative cystectomy. Updated long-term follow-up (median 74 mo) shows that significantly greater OS and DSS was maintained for patients with ATM, RB1, or FANCC mutations. The 5-yr survival rate for patients with at least one mutation was 85%, compared to 45% for patients without a mutation. On the basis of the associations with response and long-term OS and DSS, we propose that these alterations may be useful as predictive biomarkers to allow clinicians to prioritize patients who are most likely to benefit from NAC before radical cystectomy. PATIENT SUMMARY: In this report we looked at outcomes for patients with muscle-invasive bladder cancer treated with cisplatin-based chemotherapy before surgery (neoadjuvant) who had mutations in a set of DNA damage repair genes (ATM, RB1, FANCC) compared to those who did not. We found that patients who had at least one mutation in one of these genes survived longer after receiving cisplatin chemotherapy before surgery than patients who did not.
Keywords: Biomarkers; Bladder cancer; Chemotherapy; Cisplatin; DNA damage repair; Neoadjuvant.
Copyright © 2020 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer.Eur Urol. 2015 Dec;68(6):959-67. doi: 10.1016/j.eururo.2015.07.009. Epub 2015 Aug 1. Eur Urol. 2015. PMID: 26238431 Free PMC article.
-
Genomic Differences Between "Primary" and "Secondary" Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy.Eur Urol. 2019 Feb;75(2):231-239. doi: 10.1016/j.eururo.2018.09.002. Epub 2018 Oct 2. Eur Urol. 2019. PMID: 30290956 Free PMC article.
-
Phase II Trial of Risk-Enabled Therapy After Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer (RETAIN 1).J Clin Oncol. 2025 Mar 20;43(9):1113-1122. doi: 10.1200/JCO-24-01214. Epub 2024 Dec 16. J Clin Oncol. 2025. PMID: 39680823 Clinical Trial.
-
Clinical outcome in patients with locally advanced bladder carcinoma treated with conservative multimodality therapy.Urology. 2004 Sep;64(3):488-93. doi: 10.1016/j.urology.2004.04.088. Urology. 2004. PMID: 15351577 Review.
-
Forty years of cisplatin-based chemotherapy in muscle-invasive bladder cancer: are we understanding how, who and when?World J Urol. 2019 Sep;37(9):1759-1765. doi: 10.1007/s00345-018-2544-8. Epub 2018 Nov 3. World J Urol. 2019. PMID: 30392011 Review.
Cited by
-
Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted.Int J Mol Sci. 2022 Jun 29;23(13):7241. doi: 10.3390/ijms23137241. Int J Mol Sci. 2022. PMID: 35806243 Free PMC article. Review.
-
Biomarker challenges in the pursuit of personalized neoadjuvant chemotherapy for muscle-invasive bladder cancer: conclusions from SWOG S1314.Transl Androl Urol. 2024 Mar 31;13(3):458-462. doi: 10.21037/tau-23-620. Epub 2024 Mar 12. Transl Androl Urol. 2024. PMID: 38590957 Free PMC article. No abstract available.
-
Exploring novel genomic biomarkers for response and survival after neoadjuvant chemotherapy and radical cystectomy of muscle-invasive bladder cancer.ESMO Open. 2025 Aug;10(8):105512. doi: 10.1016/j.esmoop.2025.105512. Epub 2025 Jul 14. ESMO Open. 2025. PMID: 40664147 Free PMC article.
-
Whole-exome sequencing reveals potential mechanisms of drug resistance to FGFR3-TACC3 targeted therapy and subsequent drug selection: towards a personalized medicine.BMC Med Genomics. 2020 Sep 21;13(1):138. doi: 10.1186/s12920-020-00794-x. BMC Med Genomics. 2020. PMID: 32957974 Free PMC article.
-
Avelumab as neoadjuvant therapy in patients with urothelial non-metastatic muscle invasive bladder cancer: a multicenter, randomized, non-comparative, phase II study (Oncodistinct 004 - AURA trial).BMC Cancer. 2021 Dec 2;21(1):1292. doi: 10.1186/s12885-021-08990-3. BMC Cancer. 2021. PMID: 34856936 Free PMC article. Clinical Trial.
References
-
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895–1901. doi:10.1200/JCO.2013.53.2465 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

