Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping
- PMID: 32165250
- PMCID: PMC7371536
- DOI: 10.1016/j.jhep.2020.03.006
Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping
Abstract
Background & aims: Non-alcoholic fatty liver disease (NAFLD), type 2 diabetes (T2D) and obesity are epidemiologically correlated with each other but the causal inter-relationships between them remain incompletely understood. We aimed to explore the causal relationships between the 3 diseases.
Methods: Using both UK Biobank and publicly available genome-wide association study data, we performed a 2-sample bidirectional Mendelian randomization analysis to test the causal inter-relationships between NAFLD, T2D, and obesity. Transgenic mice expressing the human PNPLA3-I148M isoforms (TghPNPLA3-I148M) were used as an example to validate causal effects and explore underlying mechanisms.
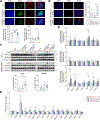
Results: Genetically driven NAFLD significantly increased the risk of T2D and central obesity but not insulin resistance or generalized obesity, while genetically driven T2D, body mass index and WHRadjBMI causally increased NAFLD risk. The animal study focusing on PNPLA3 corroborated these causal effects: compared to the TghPNPLA3-I148I controls, the TghPNPLA3-I148M mice developed glucose intolerance and increased visceral fat, but maintained normal insulin sensitivity, reduced body weight, and decreased circulating total cholesterol. Mechanistically, the TghPNPLA3-I148M mice demonstrated decreased pancreatic insulin but increased glucagon secretion, which was associated with increased pancreatic inflammation. In addition, transcription of hepatic cholesterol biosynthesis pathway genes was significantly suppressed, while transcription of thermogenic pathway genes was activated in subcutaneous and brown adipose tissues but not in visceral fat in TghPNPLA3-I148M mice.
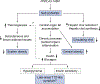
Conclusions: Our study suggests that lifelong, genetically driven NAFLD causally promotes T2D with a late-onset type 1-like diabetic subphenotype and central obesity; while genetically driven T2D, obesity, and central obesity all causally increase the risk of NAFLD. This causal relationship revealed new insights into how nature and nurture drive these diseases, providing novel hypotheses for disease subphenotyping.
Lay summary: Non-alcoholic fatty liver disease, type 2 diabetes and obesity are epidemiologically correlated with each other, but their causal relationships were incompletely understood. Herein, we identified causal relationships between these conditions, which suggest that each of these closely related diseases should be further stratified into subtypes. This is important for accurate diagnosis, prevention and treatment of these diseases.
Keywords: Mendelian randomization; Non-alcoholic fatty liver disease; Obesity; PNPLA3; Type 2 diabetes.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest All authors have reviewed the manuscript. C.W's spouse works at Regeneron Pharmaceuticals, and all other co-authors declared no conflict of interest. The sponsor of the study has no role in the study design, collection, analysis, and interpretation of data. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. - PubMed
-
- Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol 2016;11:451–496. - PubMed
-
- Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018;41:372–382. - PubMed
-
- Hu M, Phan F, Bourron O, Ferre P, Foufelle F. Steatosis and NASH in type 2 diabetes. Biochimie 2017;143:37–41. - PubMed
-
- Lonardo A, Lugari S, Ballestri S, Nascimbeni F, Baldelli E, Maurantonio M. A round trip from nonalcoholic fatty liver disease to diabetes: molecular targets to the rescue? Acta Diabetol 2019;56:385–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

