Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization
- PMID: 32165498
- PMCID: PMC7170511
- DOI: 10.1074/jbc.RA119.010002
Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization
Abstract
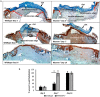
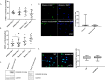
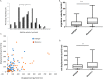
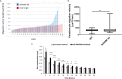
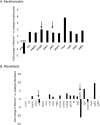
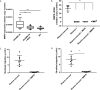
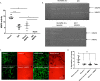
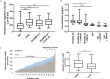
Prevention of aberrant cutaneous wound repair and appropriate regeneration of an intact and functional integument require the coordinated timing of fibroblast and keratinocyte migration. Here, we identified a mechanism whereby opposing cell-specific motogenic functions of a multifunctional intracellular and extracellular protein, the receptor for hyaluronan-mediated motility (RHAMM), coordinates fibroblast and keratinocyte migration speed and ensures appropriate timing of excisional wound closure. We found that, unlike in WT mice, in Rhamm-null mice, keratinocyte migration initiates prematurely in the excisional wounds, resulting in wounds that have re-surfaced before the formation of normal granulation tissue, leading to a defective epidermal architecture. We also noted aberrant keratinocyte and fibroblast migration in the Rhamm-null mice, indicating that RHAMM suppresses keratinocyte motility but increases fibroblast motility. This cell context-dependent effect resulted from cell-specific regulation of extracellular signal-regulated kinase 1/2 (ERK1/2) activation and expression of a RHAMM target gene encoding matrix metalloprotease 9 (MMP-9). In fibroblasts, RHAMM promoted ERK1/2 activation and MMP-9 expression, whereas in keratinocytes, RHAMM suppressed these activities. In keratinocytes, loss of RHAMM function or expression promoted epidermal growth factor receptor-regulated MMP-9 expression via ERK1/2, which resulted in cleavage of the ectodomain of the RHAMM partner protein CD44 and thereby increased keratinocyte motility. These results identify RHAMM as a key factor that integrates the timing of wound repair by controlling cell migration.
Keywords: RHAMM; cell migration; cell signaling; extracellular matrix; hyaluronan; keratinocyte; keratinocytes; wound healing; wound repair.
© 2020 Tolg et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








Similar articles
-
Rhamm-/- fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair.J Cell Biol. 2006 Dec 18;175(6):1017-28. doi: 10.1083/jcb.200511027. Epub 2006 Dec 11. J Cell Biol. 2006. PMID: 17158951 Free PMC article.
-
The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells.J Biol Chem. 2007 Jun 1;282(22):16667-80. doi: 10.1074/jbc.M702078200. Epub 2007 Mar 28. J Biol Chem. 2007. PMID: 17392272 Free PMC article.
-
A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds.Am J Pathol. 2012 Oct;181(4):1250-70. doi: 10.1016/j.ajpath.2012.06.036. Epub 2012 Aug 11. Am J Pathol. 2012. PMID: 22889846 Free PMC article.
-
Hyaluronan and RHAMM in wound repair and the "cancerization" of stromal tissues.Biomed Res Int. 2014;2014:103923. doi: 10.1155/2014/103923. Epub 2014 Aug 4. Biomed Res Int. 2014. PMID: 25157350 Free PMC article. Review.
-
Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization.Tissue Eng Part B Rev. 2022 Jun;28(3):665-676. doi: 10.1089/ten.TEB.2021.0030. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34238035 Review.
Cited by
-
The role of RHAMM in cancer: Exposing novel therapeutic vulnerabilities.Front Oncol. 2022 Aug 10;12:982231. doi: 10.3389/fonc.2022.982231. eCollection 2022. Front Oncol. 2022. PMID: 36033439 Free PMC article. Review.
-
"RHAMM knockout" mice express a truncated RHAMM protein that promotes pancreatic cancer progression with dysfunctional p53.Ann Pancreat Cancer. 2022 Jul;5:7. doi: 10.21037/apc-2022-1. Epub 2022 Jul 10. Ann Pancreat Cancer. 2022. PMID: 36507054 Free PMC article. No abstract available.
-
Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis.Cancers (Basel). 2024 May 16;16(10):1907. doi: 10.3390/cancers16101907. Cancers (Basel). 2024. PMID: 38791985 Free PMC article. Review.
-
RHAMM regulates MMTV-PyMT-induced lung metastasis by connecting STING-dependent DNA damage sensing to interferon/STAT1 pro-apoptosis signaling.Breast Cancer Res. 2023 Jun 22;25(1):74. doi: 10.1186/s13058-023-01652-1. Breast Cancer Res. 2023. PMID: 37349798 Free PMC article.
-
Hyaluronan-Mediated Motility Receptor (HMMR) Overexpression Is Correlated with Poor Survival in Patients with B-ALL.Int J Mol Sci. 2025 Jan 16;26(2):744. doi: 10.3390/ijms26020744. Int J Mol Sci. 2025. PMID: 39859458 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

