MEMPHIS: a smartphone app using psychological approaches for women with chronic pelvic pain presenting to gynaecology clinics: a randomised feasibility trial
- PMID: 32165549
- PMCID: PMC7069270
- DOI: 10.1136/bmjopen-2019-030164
MEMPHIS: a smartphone app using psychological approaches for women with chronic pelvic pain presenting to gynaecology clinics: a randomised feasibility trial
Abstract
Objectives: To evaluate the feasibility of a randomised trial of a modified, pre-existing, mindfulness meditation smartphone app for women with chronic pelvic pain.
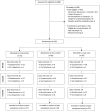
Design: Three arm randomised feasibility trial.
Setting: Women were recruited at two gynaecology clinics in the UK. Interventions were delivered via smartphone or computer at a location of participants choosing.
Participants: Women were eligible for the study if they were over 18, had been experiencing organic or non-organic chronic pelvic pain for 6 months or more, and had access to a computer or smartphone. 90 women were randomised.
Interventions: Daily mindfulness meditation delivered by smartphone app, an active control app which delivered muscle relaxation techniques, and usual care without app. Interventions were delivered over 60 days.
Primary and secondary outcome measures: Outcomes included length of recruitment, follow-up rates, adherence to the app interventions, and clinical outcomes measured at baseline, two, three and 6 months.
Results: The target sample size was recruited in 145 days. Adherence to the app interventions was extremely low (mean app use 1.8 days mindfulness meditation group, 7.0 days active control). Fifty-seven (63%) women completed 6-month follow-up, and 75 (83%) women completed at least one postrandomisation follow-up. The 95% CIs for clinical outcomes were consistent with no benefit from the mindfulness meditation app; for example, mean differences in pain acceptance scores at 60 days (higher scores are better) were -2.3 (mindfulness meditation vs usual care, 95% CI: -6.6 to 2.0) and -4.0 (mindfulness meditation vs active control, 95% CI: -8.1 to 0.1).
Conclusions: Despite high recruitment and adequate follow-up rates, demonstrating feasibility, the extremely low adherence suggests a definitive randomised trial of the mindfulness meditation app used in this study is not warranted. Future research should focus on improving patient engagement.
Trial registration numbers: NCT02721108; ISRCTN10925965; Results.
Keywords: Randomised controlled trial; chronic pain; meditation; mindfulness; mobile applications; pelvic pain.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
mHealth: providing a mindfulness app for women with chronic pelvic pain in gynaecology outpatient clinics: qualitative data analysis of user experience and lessons learnt.BMJ Open. 2020 Mar 12;10(3):e030711. doi: 10.1136/bmjopen-2019-030711. BMJ Open. 2020. PMID: 32165550 Free PMC article. Clinical Trial.
-
Smartphone App Using Mindfulness Meditation for Women With Chronic Pelvic Pain (MEMPHIS): Protocol for a Randomized Feasibility Trial.JMIR Res Protoc. 2018 Jan 15;7(1):e8. doi: 10.2196/resprot.7720. JMIR Res Protoc. 2018. PMID: 29335232 Free PMC article.
-
Effectiveness of app-based relaxation for patients with chronic low back pain (Relaxback) and chronic neck pain (Relaxneck): study protocol for two randomized pragmatic trials.Trials. 2014 Dec 15;15:490. doi: 10.1186/1745-6215-15-490. Trials. 2014. PMID: 25511185 Free PMC article. Clinical Trial.
-
Mindfulness meditation-based pain relief: a mechanistic account.Ann N Y Acad Sci. 2016 Jun;1373(1):114-27. doi: 10.1111/nyas.13153. Ann N Y Acad Sci. 2016. PMID: 27398643 Free PMC article. Review.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
E-health psychological intervention in pregnant women exposed to intimate partner violence (eIPV): A protocol for a pilot randomised controlled trial.PLoS One. 2023 Mar 17;18(3):e0282997. doi: 10.1371/journal.pone.0282997. eCollection 2023. PLoS One. 2023. PMID: 36930616 Free PMC article.
-
Current Implementation of Digital Health in Chronic Disease Management: Scoping Review.J Med Internet Res. 2024 Dec 12;26:e53576. doi: 10.2196/53576. J Med Internet Res. 2024. PMID: 39666972 Free PMC article.
-
Notifications to Improve Engagement With an Alcohol Reduction App: Protocol for a Micro-Randomized Trial.JMIR Res Protoc. 2020 Aug 7;9(8):e18690. doi: 10.2196/18690. JMIR Res Protoc. 2020. PMID: 32763878 Free PMC article.
-
Systematic Review for the Medical Applications of Meditation in Randomized Controlled Trials.Int J Environ Res Public Health. 2022 Jan 22;19(3):1244. doi: 10.3390/ijerph19031244. Int J Environ Res Public Health. 2022. PMID: 35162267 Free PMC article.
-
New Evidence in the Booming Field of Online Mindfulness: An Updated Meta-analysis of Randomized Controlled Trials.JMIR Ment Health. 2021 Jul 19;8(7):e28168. doi: 10.2196/28168. JMIR Ment Health. 2021. PMID: 34279240 Free PMC article. Review.
References
-
- Moore SJ KS. Green Top Guideline No 41: the initial management of Chronic Pelvic Pain : Gynaecologists TrcoOa. In, 2012.
-
- Peters AA, van Dorst E, Jellis B, et al. . A randomized clinical trial to compare two different approaches in women with chronic pelvic pain. Obstet Gynecol 1991;77:740–4. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
