Liquid-liquid phase separation drives skin barrier formation
- PMID: 32165560
- PMCID: PMC7258523
- DOI: 10.1126/science.aax9554
Liquid-liquid phase separation drives skin barrier formation
Abstract
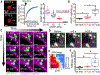
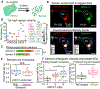
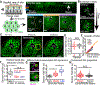
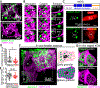
At the body surface, skin's stratified squamous epithelium is challenged by environmental extremes. The surface of the skin is composed of enucleated, flattened surface squames. They derive from underlying, transcriptionally active keratinocytes that display filaggrin-containing keratohyalin granules (KGs) whose function is unclear. Here, we found that filaggrin assembles KGs through liquid-liquid phase separation. The dynamics of phase separation governed terminal differentiation and were disrupted by human skin barrier disease-associated mutations. We used fluorescent sensors to investigate endogenous phase behavior in mice. Phase transitions during epidermal stratification crowded cellular spaces with liquid-like KGs whose coalescence was restricted by keratin filament bundles. We imaged cells as they neared the skin surface and found that environmentally regulated KG phase dynamics drive squame formation. Thus, epidermal structure and function are driven by phase-separation dynamics.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Liquid droplets in the skin.Science. 2020 Mar 13;367(6483):1193-1194. doi: 10.1126/science.abb0060. Science. 2020. PMID: 32165570 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

