Structure of V-ATPase from the mammalian brain
- PMID: 32165585
- PMCID: PMC7324285
- DOI: 10.1126/science.aaz2924
Structure of V-ATPase from the mammalian brain
Abstract
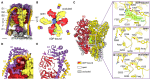
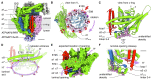
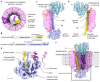
In neurons, the loading of neurotransmitters into synaptic vesicles uses energy from proton-pumping vesicular- or vacuolar-type adenosine triphosphatases (V-ATPases). These membrane protein complexes possess numerous subunit isoforms, which complicates their analysis. We isolated homogeneous rat brain V-ATPase through its interaction with SidK, a Legionella pneumophila effector protein. Cryo-electron microscopy allowed the construction of an atomic model, defining the enzyme's ATP:proton ratio as 3:10 and revealing a homolog of yeast subunit f in the membrane region, which we tentatively identify as RNAseK. The c ring encloses the transmembrane anchors for cleaved ATP6AP1/Ac45 and ATP6AP2/PRR, the latter of which is the (pro)renin receptor that, in other contexts, is involved in both Wnt signaling and the renin-angiotensin system that regulates blood pressure. This structure shows how ATP6AP1/Ac45 and ATP6AP2/PRR enable assembly of the enzyme's catalytic and membrane regions.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
-
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. - PubMed
-
- Kane PM. Disassembly and reassembly of the yeast vacuolar H(+)-ATPase in vivo. J Biol Chem. 1995;270:17025–17032. - PubMed
-
- Sumner JP, Dow JA, Earley FG, Klein U, Jager D, Wieczorek H. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem. 1995;270:5649–5653. - PubMed
-
- Bodzeta A, Kahms M, Klingauf J. The Presynaptic v-ATPase Reversibly Disassembles and Thereby Modulates Exocytosis but Is Not Part of the Fusion Machinery. Cell Rep. 2017;20:1348–1359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

