Cholesterol-dependent enrichment of understudied erythrocytic stages of human Plasmodium parasites
- PMID: 32165667
- PMCID: PMC7067793
- DOI: 10.1038/s41598-020-61392-6
Cholesterol-dependent enrichment of understudied erythrocytic stages of human Plasmodium parasites
Abstract
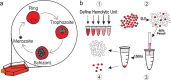
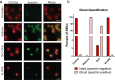
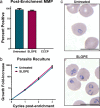
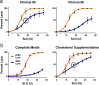
For intracellular pathogens, the host cell provides needed protection and nutrients. A major challenge of intracellular parasite research is collection of high parasite numbers separated from host contamination. This situation is exemplified by the malaria parasite, which spends a substantial part of its life cycle inside erythrocytes as rings, trophozoites, and schizonts, before egress and reinvasion. Erythrocytic Plasmodium parasite forms refractory to enrichment remain understudied due to high host contamination relative to low parasite numbers. Here, we present a method for separating all stages of Plasmodium-infected erythrocytes through lysis and removal of uninfected erythrocytes. The Streptolysin O-Percoll (SLOPE) method is effective on previously inaccessible forms, including circulating rings from malaria-infected patients and artemisinin-induced quiescent parasites. SLOPE can be used on multiple parasite species, under multiple media formulations, and lacks measurable impacts on parasite viability. We demonstrate erythrocyte membrane cholesterol levels modulate the preferential lysis of uninfected host cells by SLO, and therefore modulate the effectiveness of SLOPE. Targeted metabolomics of SLOPE-enriched ring stage samples confirms parasite-derived metabolites are increased and contaminating host material is reduced compared to non-enriched samples. Due to consumption of cholesterol by other intracellular bacteria and protozoa, SLOPE holds potential for improving research on organisms beyond Plasmodium.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Organization, W. H. World Malaria Report 2018. (WHO, Geneva, Switzerland, 2018).
-
- WHO. Guidelines for the treatment of malaria. (Geneva, Switzerland, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

