Identification of highly potent and selective inhibitor, TIPTP, of the p22phox-Rubicon axis as a therapeutic agent for rheumatoid arthritis
- PMID: 32165681
- PMCID: PMC7067850
- DOI: 10.1038/s41598-020-61630-x
Identification of highly potent and selective inhibitor, TIPTP, of the p22phox-Rubicon axis as a therapeutic agent for rheumatoid arthritis
Abstract
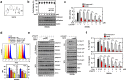
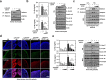
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease linked to oxidative stress, which is associated with significant morbidity. The NADPH oxidase complex (NOX) produces reactive oxygen species (ROS) that are among the key markers for determining RA's pathophysiology. Therefore, understanding ROS-regulated molecular pathways and their interaction is necessary for developing novel therapeutic approaches for RA. Here, by combining mouse genetics and biochemistry with clinical tissue analysis, we reveal that in vivo Rubicon interacts with the p22phox subunit of NOX, which is necessary for increased ROS-mediated RA pathogenesis. Furthermore, we developed a series of new aryl propanamide derivatives consisting of tetrahydroindazole and thiadiazole as p22phox inhibitors and selected 2-(tetrahydroindazolyl)phenoxy-N-(thiadiazolyl)propanamide 2 (TIPTP, M.W. 437.44), which showed considerably improved potency, reaching an IC50 value up to 100-fold lower than an inhibitor that we previously synthesized reported N8 peptide-mimetic small molecule (blocking p22phox-Rubicon interaction). Notably, TIPTP treatment showed significant therapeutic effects a mouse model for RA. Furthermore, TIPTP had anti-inflammatory effects ex vivo in monocytes from healthy individuals and synovial fluid cells from RA patients. These findings may have clinical applications for the development of TIPTP as a small molecule inhibitor of the p22phox-Rubicon axis for the treatment of ROS-driven diseases such as RA.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

