Formation of distinct prion protein amyloid fibrils under identical experimental conditions
- PMID: 32165692
- PMCID: PMC7067779
- DOI: 10.1038/s41598-020-61663-2
Formation of distinct prion protein amyloid fibrils under identical experimental conditions
Abstract
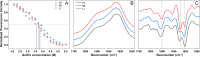
Protein aggregation into amyloid fibrils is linked to multiple neurodegenerative disorders, such as Alzheimer's, Parkinson's or Creutzfeldt-Jakob disease. A better understanding of the way these aggregates form is vital for the development of drugs. A large detriment to amyloid research is the ability of amyloidogenic proteins to spontaneously aggregate into multiple structurally distinct fibrils (strains) with different stability and seeding properties. In this work we show that prion proteins are capable of forming more than one type of fibril under the exact same conditions by assessing their Thioflavin T (ThT) binding ability, morphology, secondary structure, stability and seeding potential.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

