Hyperglycaemia does not increase perfusion deficits after focal cerebral ischaemia in male Wistar rats
- PMID: 32166145
- PMCID: PMC7058243
- DOI: 10.1177/2398212818794820
Hyperglycaemia does not increase perfusion deficits after focal cerebral ischaemia in male Wistar rats
Abstract
Background: Hyperglycaemia is associated with a worse outcome in acute ischaemic stroke patients; yet the pathophysiological mechanisms of hyperglycaemia-induced damage are poorly understood. We hypothesised that hyperglycaemia at the time of stroke onset exacerbates ischaemic brain damage by increasing the severity of the blood flow deficit.
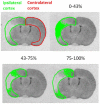
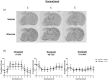
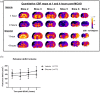
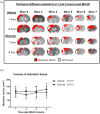
Methods: Adult, male Wistar rats were randomly assigned to receive vehicle or glucose solutions prior to permanent middle cerebral artery occlusion. Cerebral blood flow was assessed semi-quantitatively either 1 h after middle cerebral artery occlusion using 99mTc-D, L-hexamethylpropyleneamine oxime (99mTc-HMPAO) autoradiography or, in a separate study, using quantitative pseudo-continuous arterial spin labelling for 4 h after middle cerebral artery occlusion. Diffusion weighted imaging was performed alongside pseudo-continuous arterial spin labelling and acute lesion volumes calculated from apparent diffusion coefficient maps. Infarct volume was measured at 24 h using rapid acquisition with refocused echoes T2-weighted magnetic resonance imaging.
Results: Glucose administration had no effect on the severity of ischaemia when assessed by either 99mTc-HMPAO autoradiography or pseudo-continuous arterial spin labelling perfusion imaging. In comparison to the vehicle group, apparent diffusion coefficient-derived lesion volume 2-4 h post-middle cerebral artery occlusion and infarct volume 24 h post-middle cerebral artery occlusion were significantly greater in the glucose group.
Conclusions: Hyperglycaemia increased acute lesion and infarct volumes but there was no evidence that the acute blood flow deficit was exacerbated. The data reinforce the conclusion that the detrimental effects of hyperglycaemia are rapid, and that treatment of post-stroke hyperglycaemia in the acute period is essential but the mechanisms of hyperglycaemia-induced harm remain unclear.
Keywords: Cerebral blood flow; animal model; focal ischaemia; hyperglycaemia; magnetic resonance imaging.
© The Author(s) 2018.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures


References
-
- Anderson RE, Tan WK, Martin HS, et al. (1999) Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke 30(1): 160–170. - PubMed
-
- Bartus RT, Dean RL, Cavanaugh K, et al. (1995) Time-related neuronal changes following middle cerebral artery occlusion: Implications for therapeutic intervention and the role of calpain. Journal of Cerebral Blood Flow & Metabolism 15(6): 969–979. - PubMed
-
- Campbell BC, Macrae IM. (2015) Translational perspectives on perfusion-diffusion mismatch in ischemic stroke. International Journal of Stroke 10(2): 163–162. - PubMed
LinkOut - more resources
Full Text Sources

