Incidence of Dexmedetomidine Withdrawal in Adult Critically Ill Patients: A Pilot Study
- PMID: 32166276
- PMCID: PMC7063945
- DOI: 10.1097/CCE.0000000000000035
Incidence of Dexmedetomidine Withdrawal in Adult Critically Ill Patients: A Pilot Study
Abstract
To determine the incidence of dexmedetomidine withdrawal in adult critically ill patients.
Design: This was a prospective, observational study of patients from November 2017 to December 2018.
Setting: Medical-surgical, cardiothoracic, and neurosurgical ICUs in a tertiary care hospital.
Patients: Adult critically ill patients on dexmedetomidine infusions for at least 3 days.
Interventions: Indicators of withdrawal were assessed at baseline and at least daily during the dexmedetomidine wean period. Delirium was assessed using the Confusion Assessment Method for the ICU. Sedation was assessed using the Richmond Agitation-Sedation Scale. The Withdrawal Assessment Tool-1 was performed and vital signs were recorded during each assessment. Patients were considered positive for dexmedetomidine withdrawal if they had two or more of the following symptoms: positive Confusion Assessment Method for the ICU, Richmond Agitation-Sedation Scale greater than +1, positive Withdrawal Assessment Tool-1 assessment, tachycardia (heart rate > 90 beats/min), and hypertension (systolic blood pressure > 140 mm Hg or mean arterial pressure > 90).
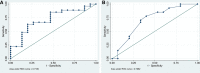
Measurements and main results: Forty-two patients were included in the study, with 64% of patients experiencing signs of dexmedetomidine withdrawal. The median time on dexmedetomidine for all patients was 9.6 days (5.8-12.7 d), and the median dose of dexmedetomidine received was 0.8 µg/kg/hr (0.5-1 µg/kg/hr). Of the patients who were positive for withdrawal, the most prevalent withdrawal symptoms observed included delirium, hypertension, and agitation (93%, 48%, and 33%, respectively). We found no correlation between chronic opioid tolerance and incidence of withdrawal symptoms. Peak dexmedetomidine doses greater than 0.8 µg/kg/hr and cumulative daily doses of dexmedetomidine greater than 12.9 µg/kg/d were associated with a higher incidence of withdrawal.
Conclusions: The majority of patients in our study demonstrated signs that may be indicative of dexmedetomidine withdrawal. Peak and cumulative daily dexmedetomidine dose, rather than duration of therapy, may be associated with a higher incidence of withdrawal signs. Regular screening of patients on prolonged dexmedetomidine infusions is recommended to ensure safe and effective use in critically ill patients.
Keywords: critical care; critically ill; dexmedetomidine; intensive care unit; sedation; withdrawal.
Copyright © 2019 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Precedex(TM) Intravenous Injection, Dexmedetomidine HCl Intravenous Injection. Lake Forest, IL: Hospira, Inc; 2016. per FDA.
-
- Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 20072982644–2653 - PubMed
-
- Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA 2009301489–499 - PubMed
-
- Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 20123071151–1160 - PubMed
-
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 201846e825–e873 - PubMed
LinkOut - more resources
Full Text Sources

