A Case Series of Vaping-Associated Lung Injury Requiring Mechanical Ventilation
- PMID: 32166299
- PMCID: PMC7063900
- DOI: 10.1097/CCE.0000000000000079
A Case Series of Vaping-Associated Lung Injury Requiring Mechanical Ventilation
Abstract
Objectives: Vaping-associated lung injury has rapidly become a nationwide epidemic and a threat to public health. In this case series, we describe unique clinical features of severe vaping-associated lung injury, defined as respiratory failure due to vaping that requires mechanical ventilation.
Data sources: Clinical observation of four patients.
Study selection: Case series.
Data extraction: Data and images were extract from medical records after approval was obtained from the institutional review board.
Data synthesis: Four patients were admitted to the ICU with severe manifestation of vaping-associated lung injury. Although every case required mechanical ventilatory support (venovenous extracorporeal membrane oxygenation in one patient), all patients survived and were discharged without supplemental oxygen. Systemic corticosteroids were administered in three patients and N-acetyl cysteine in one. A postdischarge pulmonary function test in one patient was normal except for mildly decreased diffusing capacity.
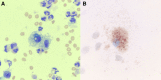
Conclusions: Based on our experience, prognosis of severe vaping-associated lung injury appears favorable with aggressive supportive care, although there is evidence from existing literature that mortality rate might rise with increasing disease severity. Underlying mechanism of lung injury might be similar between vaping-associated lung injury and amiodarone pneumonitis. Foamy or lipid-laden macrophages, seen in both conditions, might be a marker of cytotoxicity from substances contained in e-cigarettes, such as vitamin E acetate. Systemic corticosteroids, and possibly N-acetyl cysteine, could be considered as therapeutic adjuncts in vaping-associated lung injury. Serial pulmonary function tests should be obtained in these patients to monitor for potential long-term complications. The primary limitations of this case series are its small sample and lack of longitudinal follow-up data.
Keywords: N-acetyl cysteine; acute lung injury; alveolar macrophages; bronchoalveolar lavage; pulmonary function test; vaping.
Copyright © 2020 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Drs. Chen and Parimon are researchers funded by the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - preliminary report. N Engl J Med. 2019 Sep 6. [Epub ahead of print] - PubMed
-
- Centers for Disease Control and Prevention: Outbreak of Lung Injury Associated With E-Cigarette Use, or Vaping. 2019.. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-d.... Accessed November 10, 2019
-
- Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019; 381:1780–1781 - PubMed
-
- Christiani DC. Vaping-induced lung injury. N Engl J Med. 2019 Sep 6. [Epub ahead of print] - PubMed
Publication types
LinkOut - more resources
Full Text Sources

