Neurochemical Characterization of Brainstem Pro-Opiomelanocortin Cells
- PMID: 32166324
- PMCID: PMC7102873
- DOI: 10.1210/endocr/bqaa032
Neurochemical Characterization of Brainstem Pro-Opiomelanocortin Cells
Abstract
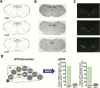
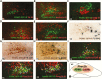
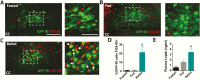
Genetic research has revealed pro-opiomelanocortin (POMC) to be a fundamental regulator of energy balance and body weight in mammals. Within the brain, POMC is primarily expressed in the arcuate nucleus of the hypothalamus (ARC), while a smaller population exists in the brainstem nucleus of the solitary tract (POMCNTS). We performed a neurochemical characterization of this understudied population of POMC cells using transgenic mice expressing green fluorescent protein (eGFP) under the control of a POMC promoter/enhancer (PomceGFP). Expression of endogenous Pomc mRNA in the nucleus of the solitary tract (NTS) PomceGFP cells was confirmed using fluorescence-activating cell sorting (FACS) followed by quantitative PCR. In situ hybridization histochemistry of endogenous Pomc mRNA and immunohistochemical analysis of eGFP revealed that POMC is primarily localized within the caudal NTS. Neurochemical analysis indicated that POMCNTS is not co-expressed with tyrosine hydroxylase (TH), glucagon-like peptide 1 (GLP-1), cholecystokinin (CCK), brain-derived neurotrophic factor (BDNF), nesfatin, nitric oxide synthase 1 (nNOS), seipin, or choline acetyltransferase (ChAT) cells, whereas 100% of POMCNTS is co-expressed with transcription factor paired-like homeobox2b (Phox2b). We observed that 20% of POMCNTS cells express receptors for adipocyte hormone leptin (LepRbs) using a PomceGFP:LepRbCre:tdTOM double-reporter line. Elevations in endogenous or exogenous leptin levels increased the in vivo activity (c-FOS) of a small subset of POMCNTS cells. Using ex vivo slice electrophysiology, we observed that this effect of leptin on POMCNTS cell activity is postsynaptic. These findings reveal that a subset of POMCNTS cells are responsive to both changes in energy status and the adipocyte hormone leptin, findings of relevance to the neurobiology of obesity.
Keywords: NTS; Pomc; leptin receptor; obesity.
© Endocrine Society 2020.
Figures

Similar articles
-
Characterization of leptin-responsive neurons in the caudal brainstem.Endocrinology. 2006 Jul;147(7):3190-5. doi: 10.1210/en.2005-0877. Epub 2006 Apr 6. Endocrinology. 2006. PMID: 16601142
-
Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract.Endocrinology. 2012 Oct;153(10):4600-7. doi: 10.1210/en.2012-1282. Epub 2012 Aug 6. Endocrinology. 2012. PMID: 22869346 Free PMC article.
-
Phenotype of neurons in the nucleus of the solitary tract that express CCK-induced activation of the ERK signaling pathway.Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R845-54. doi: 10.1152/ajpregu.90531.2008. Epub 2009 Jan 28. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19176891 Free PMC article.
-
Electrophysiological actions of peripheral hormones on melanocortin neurons.Ann N Y Acad Sci. 2003 Jun;994:175-86. doi: 10.1111/j.1749-6632.2003.tb03178.x. Ann N Y Acad Sci. 2003. PMID: 12851314 Review.
-
Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes.Biochem J. 2010 May 27;428(3):305-24. doi: 10.1042/BJ20091957. Biochem J. 2010. PMID: 20504281 Review.
Cited by
-
POMC neuronal heterogeneity in energy balance and beyond: an integrated view.Nat Metab. 2021 Mar;3(3):299-308. doi: 10.1038/s42255-021-00345-3. Epub 2021 Feb 25. Nat Metab. 2021. PMID: 33633406 Free PMC article. Review.
-
NPAS4 Depletion in POMC Neurons Protects From Obesity and Alters the Feeding-regulated Transcriptome in Male Mice.Endocrinology. 2025 May 19;166(7):bqaf083. doi: 10.1210/endocr/bqaf083. Endocrinology. 2025. PMID: 40296822 Free PMC article.
-
Identification of AgRP cells in the murine hindbrain that drive feeding.Mol Metab. 2024 Feb;80:101886. doi: 10.1016/j.molmet.2024.101886. Epub 2024 Jan 19. Mol Metab. 2024. PMID: 38246589 Free PMC article.
-
SK3 in POMC neurons plays a sexually dimorphic role in energy and glucose homeostasis.Cell Biosci. 2022 Oct 9;12(1):170. doi: 10.1186/s13578-022-00907-2. Cell Biosci. 2022. PMID: 36210455 Free PMC article.
-
A survey of the mouse hindbrain in the fed and fasted states using single-nucleus RNA sequencing.Mol Metab. 2021 Nov;53:101240. doi: 10.1016/j.molmet.2021.101240. Epub 2021 May 4. Mol Metab. 2021. PMID: 33962048 Free PMC article.
References
-
- Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20(5):203–215. - PubMed
-
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4(6):605–611. - PubMed
-
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141(9):3072–3079. - PubMed
-
- Huszar D, Lynch CA, Fairchild-Huntress V, et al. . Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT098012/WT_/Wellcome Trust/United Kingdom
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
- BB/K001418/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- 100574/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- MRC_MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/NO17838/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 093566/Z/10/A/WT_/Wellcome Trust/United Kingdom
- MC/PC/15077/MRC_/Medical Research Council/United Kingdom
- 204815/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- WT081713/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

