Melanocortin therapy ameliorates podocytopathy and proteinuria in experimental focal segmental glomerulosclerosis involving a podocyte specific non-MC1R-mediated melanocortinergic signaling
- PMID: 32167144
- PMCID: PMC9870294
- DOI: 10.1042/CS20200016
Melanocortin therapy ameliorates podocytopathy and proteinuria in experimental focal segmental glomerulosclerosis involving a podocyte specific non-MC1R-mediated melanocortinergic signaling
Abstract
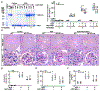
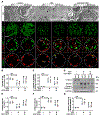
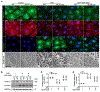
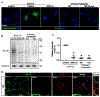
The clinical effectiveness of adrenocorticotropin in inducing remission of steroid-resistant nephrotic syndrome points to a steroidogenic-independent anti-proteinuric activity of melanocortins. However, which melanocortin receptors (MCR) convey this beneficial effect and if systemic or podocyte-specific mechanisms are involved remain uncertain. In vivo, wild-type (WT) mice developed heavy proteinuria and kidney dysfunction following Adriamycin insult, concomitant with focal segmental glomerulosclerosis (FSGS) and podocytopathy, marked by loss of podocin and synaptopodin, podocytopenia and extensive foot process effacement on electron microscopy. All these pathologic findings were prominently attenuated by NDP-MSH, a potent non-steroidogenic pan-MCR agonist. Surprisingly, MC1R deficiency in MC1R-null mice barely affected the severity of Adriamycin-elicited injury. Moreover, the beneficial effect of NDP-MSH was completely preserved in MC1R-null mice, suggesting that MC1R is likely non-essential for the protective action. A direct podocyte effect seems to contribute to the beneficial effect of NDP-MSH, because Adriamycin-inflicted cytopathic signs in primary podocytes prepared from WT mice were all mitigated by NDP-MSH, including apoptosis, loss of podocyte markers, de novo expression of the podocyte injury marker desmin, actin cytoskeleton derangement and podocyte hypermotility. Consistent with in vivo findings, the podoprotective activity of NDP-MSH was fully preserved in MC1R-null podocytes. Mechanistically, MC1R expression was predominantly distributed to glomerular endothelial cells in glomeruli but negligibly noted in podocytes in vivo and in vitro, suggesting that MC1R signaling is unlikely involved in direct podocyte protection. Ergo, melanocortin therapy protects against podocyte injury and ameliorates proteinuria and glomerulopathy in experimental FSGS, at least in part, via a podocyte-specific non-MC1R-mediated melanocortinergic signaling.
Keywords: adrenocorticotropic hormone; apoptosis; cytoskeleton; glomerular disease; podocytes.
© 2020 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Figures


References
-
- D’Agati VD, Kaskel FJ and Falk RJ (2011) Focal segmental glomerulosclerosis. The New England journal of medicine. 365, 2398–2411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

