Disinfection of contaminated metal implants with an Er:YAG laser
- PMID: 32167192
- PMCID: PMC7687249
- DOI: 10.1002/jor.24662
Disinfection of contaminated metal implants with an Er:YAG laser
Abstract
Infections related to orthopedic procedures are considered particularly severe when implantation materials are used, because effective treatments for biofilm removal are lacking. In this study, the relatively new approach for infection control by using an erbium:yttrium-aluminum-garnet (Er:YAG) laser was tested. This laser vaporizes all water containing cells in a very effective, precise, and predictable manner and results in only minimal thermal damage. For preliminary testing, 42 steel plates and 42 pins were seeded with mixed cultures. First, the minimally necessary laser energy for biofilm removal was determined. Subsequently, the effectiveness of biofilm removal with the Er:YAG laser and the cleansing of the metal implants with octenidine-soaked gauze was compared. Then, we compared the effectiveness of biofilm removal on 207 steel pins from 41 patients directly after explantation. Sonication and scanning electron microscopy were used for analysis. Laser fluences exceeding 2.8 J/cm2 caused a complete extinction of all living cells by a single-laser impulse. Cleansing with octenidine-soaked gauze and irradiation with the Er:YAG laser are both thoroughly effective when applied to seeded pins. In contrast, when explanted pins with fully developed biofilms were analyzed, we found a significant advantage of the laser procedure. The Er:YAG laser offers a secure, complete, and nontoxic eradication of all kinds of pathogens from metal implants without damaging the implant and without the possible development of resistance. The precise noncontact removal of adjacent tissue is a decisive advantage over conventional disinfectants. Therefore, laser irradiation could become a valuable method in every debridement, antibiotics, and implant retention procedure.
Keywords: Er:YAG laser; biofilm removal, DAIR; implant-related infection; laser disinfection; pin-tract infection.
© 2020 The Authors. Journal of Orthopaedic Research® published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures



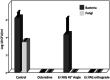
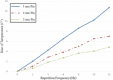



References
-
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic‐joint infections. N Engl J Med. 2004;351(16):1645‐1654. - PubMed
-
- Gristina AG. Biomaterial‐centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588‐1595. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318‐1322. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

