Flow-regulated endothelial glycocalyx determines metastatic cancer cell activity
- PMID: 32167209
- PMCID: PMC7200301
- DOI: 10.1096/fj.201901920R
Flow-regulated endothelial glycocalyx determines metastatic cancer cell activity
Abstract
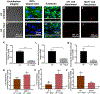
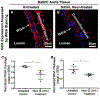
Cancer metastasis and secondary tumor initiation largely depend on circulating tumor cell (CTC) and vascular endothelial cell (EC) interactions by incompletely understood mechanisms. Endothelial glycocalyx (GCX) dysfunction may play a significant role in this process. GCX structure depends on vascular flow patterns, which are irregular in tumor environments. This work presents evidence that disturbed flow (DF) induces GCX degradation, leading to CTC homing to the endothelium, a first step in secondary tumor formation. A 2-fold greater attachment of CTCs to human ECs was found to occur under DF conditions, compared to uniform flow (UF) conditions. These results corresponded to an approximately 50% decrease in wheat germ agglutinin (WGA)-labeled components of the GCX under DF conditions, vs UF conditions, with undifferentiated levels of CTC-recruiting E-selectin under DF vs UF conditions. Confirming the role of the GCX, neuraminidase induced the degradation of WGA-labeled GCX under UF cell culture conditions or in Balb/C mice and led to an over 2-fold increase in CTC attachment to ECs or Balb/C mouse lungs, respectively, compared to untreated conditions. These experiments confirm that flow-induced GCX degradation can enable metastatic CTC arrest. This work, therefore, provides new insight into pathways of secondary tumor formation.
Keywords: disturbed flow; endothelial cells; glycocalyx; intercellular interactions; metastatic cancer cells.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Figures





References
-
- De Souza LM, Robertson BM, and Robertson GP, Future of circulating tumor cells in the melanoma clinical and research laboratory settings. Cancer Lett, 2017. 392: p. 60–70. - PubMed
-
- Cegan M, Kobierzycki C, Kolostova K, Kiss I, Bobek V, and Grill R, Circulating tumor cells in urological cancers. Folia Histochem Cytobiol, 2017. 55(3): p. 107–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

