Photodynamic Control of the Chain Length in Supramolecular Polymers: Switching an Intercalator into a Chain Capper
- PMID: 32167302
- PMCID: PMC7118707
- DOI: 10.1021/jacs.0c00858
Photodynamic Control of the Chain Length in Supramolecular Polymers: Switching an Intercalator into a Chain Capper
Abstract
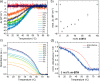
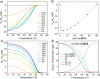
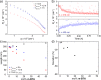
Supramolecular systems are intrinsically dynamic and sensitive to changes in molecular structure and external conditions. Because of these unique properties, strategies to control polymer length, composition, comonomer sequence, and morphology have to be developed for sufficient control over supramolecular copolymerizations. We designed photoresponsive, mono acyl hydrazone functionalized benzene-1,3,5-tricarboxamide (m-BTA) monomers that play a dual role in the coassembly with achiral alkyl BTAs (a-BTA). In the E isomer form, the chiral m-BTA monomers intercalate into stacks of a-BTA and dictate the chirality of the helices. Photoisomerization to the Z isomer transforms the intercalator into a chain capper, allowing dynamic shortening of chain length in the supramolecular aggregates. We combine optical spectroscopy and light-scattering experiments with theoretical modeling to show the reversible decrease in length when switching from the E to Z isomer of m-BTA in the copolymer with inert a-BTA. With a mass-balance thermodynamic model, we gain additional insights into the composition of copolymers and length distributions of the species over a broad range of concentrations and mixing ratios of a-BTA/m-BTA. Moreover, the model was used to predict the impact of an additive (chain capper and intercalator) on the chain length over a range of concentrations, showing a remarkable amplification of efficiency at high concentrations. By employing a stimuli-responsive comonomer in a mostly inert polymer, we can cooperatively amplify the effect of the switching and obtain photocontrol of polymer length. Moreover, this dynamic decrease in chain length causes a macroscopic gel-to-sol phase transformation of the copolymer gel, although 99.4% of the organogel is inert to the light stimulus.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

