Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities
- PMID: 32168344
- PMCID: PMC7069647
- DOI: 10.1371/journal.pone.0224052
Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities
Abstract
Background: Codeine, a 3-methylmorphine, and other related opioids have been implicated in androgen suppression, although the associated mechanisms remain unclear.
Aim: Therefore, the objective of the current study was to elucidate the in vivo molecular mechanisms underlying codeine-induced androgen suppression.
Methods: This study made use of twenty-one healthy male rabbits, distributed into three groups randomly, control and codeine-treated groups. The control had 1ml of normal saline daily p.o. The codeine-treated groups received either 4mg/kg b.w of codeine or 10mg/kg b.w of codeine p.o. for six weeks. Reproductive hormonal profile, testicular weight, testicular enzymes, oxidative and inflammatory parameters, testicular DNA fragmentation, histological examination and apoptosis marker were evaluated to examine the effects of codeine use.
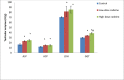
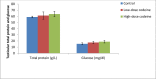
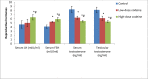
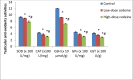
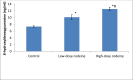
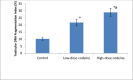
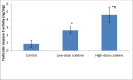
Key findings: Oral administration of codeine resulted in testicular atrophy and alterations in testicular histomorphology, elevated testicular enzymes, and suppression of circulatory and intra-testicular testosterone. These changes were associated with a marked rise in oxidative markers and decline in the activities of testicular enzymatic antioxidants, as well as oxidative DNA damage, inflammatory response, testicular DNA fragmentation, and caspase-dependent apoptosis (p<0.05).
Significance: In conclusion, chronic codeine use resulted in testicular degeneration and testosterone suppression, which is attributable to TNF-α/nitric oxide-/oxidative stress-mediated caspase-dependent apoptotic testicular cell death and loss of testicular function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Codeine-induced hepatic injury is via oxido-inflammatory damage and caspase-3-mediated apoptosis.Mol Biol Rep. 2020 Dec;47(12):9521-9530. doi: 10.1007/s11033-020-05983-6. Epub 2020 Nov 19. Mol Biol Rep. 2020. PMID: 33211294
-
Codeine-induced sperm DNA damage is mediated predominantly by oxidative stress rather than apoptosis.Redox Rep. 2020 Dec;25(1):33-40. doi: 10.1080/13510002.2020.1752003. Redox Rep. 2020. PMID: 32290793 Free PMC article.
-
L-arginine attenuates dichlorvos-induced testicular toxicity in male Wistar rats by suppressing oxidative stress-dependent activation of caspase 3-mediated apoptosis.Biomed Pharmacother. 2024 Sep;178:117136. doi: 10.1016/j.biopha.2024.117136. Epub 2024 Jul 26. Biomed Pharmacother. 2024. PMID: 39067166
-
Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators.Ecotoxicol Environ Saf. 2019 Oct 30;182:109398. doi: 10.1016/j.ecoenv.2019.109398. Epub 2019 Jul 2. Ecotoxicol Environ Saf. 2019. PMID: 31276887
-
Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats.Life Sci. 2015 Nov 15;141:13-9. doi: 10.1016/j.lfs.2015.09.015. Epub 2015 Sep 25. Life Sci. 2015. PMID: 26407473
Cited by
-
Histopathological evaluation of infertility: Lessons from laboratory rodents.Histol Histopathol. 2024 Jun;39(6):671-690. doi: 10.14670/HH-18-684. Epub 2023 Dec 1. Histol Histopathol. 2024. PMID: 38088133 Review.
-
Melatonin protect against pregabalin-induced gonadotoxicity via anti-oxidative, anti-inflammatory, anti-apoptotic, enzymatic and hormonal regulatory mechanisms in rats.BMC Pharmacol Toxicol. 2025 Feb 12;26(1):30. doi: 10.1186/s40360-025-00863-w. BMC Pharmacol Toxicol. 2025. PMID: 39940050 Free PMC article.
-
L-Arginine reverses maternal and pre-pubertal codeine exposure-induced sexual dysfunction via upregulation of androgen receptor gene and NO/cGMP signaling.PLoS One. 2022 Sep 13;17(9):e0274411. doi: 10.1371/journal.pone.0274411. eCollection 2022. PLoS One. 2022. PMID: 36099318 Free PMC article.
-
Impact of hypoxia on male reproductive functions.Mol Cell Biochem. 2023 Apr;478(4):875-885. doi: 10.1007/s11010-022-04559-1. Epub 2022 Sep 15. Mol Cell Biochem. 2023. PMID: 36107286 Review.
-
Psychoactive drugs and male fertility: impacts and mechanisms.Reprod Biol Endocrinol. 2023 Jul 28;21(1):69. doi: 10.1186/s12958-023-01098-2. Reprod Biol Endocrinol. 2023. PMID: 37507788 Free PMC article. Review.
References
-
- Aloisi AM, Aurilio C, Bachiocco V, Biasi G, Fiorenzani P, Pace MC, et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology. 2009;34(Suppl 1):S162–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

