Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics
- PMID: 32168372
- PMCID: PMC7187988
- DOI: 10.1042/BCJ20190468
Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics
Abstract
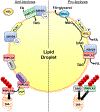
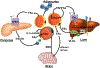
Fatty acids (FAs) are stored safely in the form of triacylglycerol (TAG) in lipid droplet (LD) organelles by professional storage cells called adipocytes. These lipids are mobilized during adipocyte lipolysis, the fundamental process of hydrolyzing TAG to FAs for internal or systemic energy use. Our understanding of adipocyte lipolysis has greatly increased over the past 50 years from a basic enzymatic process to a dynamic regulatory one, involving the assembly and disassembly of protein complexes on the surface of LDs. These dynamic interactions are regulated by hormonal signals such as catecholamines and insulin which have opposing effects on lipolysis. Upon stimulation, patatin-like phospholipase domain containing 2 (PNPLA2)/adipocyte triglyceride lipase (ATGL), the rate limiting enzyme for TAG hydrolysis, is activated by the interaction with its co-activator, alpha/beta hydrolase domain-containing protein 5 (ABHD5), which is normally bound to perilipin 1 (PLIN1). Recently identified negative regulators of lipolysis include G0/G1 switch gene 2 (G0S2) and PNPLA3 which interact with PNPLA2 and ABHD5, respectively. This review focuses on the dynamic protein-protein interactions involved in lipolysis and discusses some of the emerging concepts in the control of lipolysis that include allosteric regulation and protein turnover. Furthermore, recent research demonstrates that many of the proteins involved in adipocyte lipolysis are multifunctional enzymes and that lipolysis can mediate homeostatic metabolic signals at both the cellular and whole-body level to promote inter-organ communication. Finally, adipocyte lipolysis is involved in various diseases such as cancer, type 2 diabetes and fatty liver disease, and targeting adipocyte lipolysis is of therapeutic interest.
Keywords: adipocyte; diabetes; fatty acid; lipolysis; non alcoholic fatty liver disease.
© 2020 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Figures



References
-
- Edens NK, Leibel RL, Hirsch J. Mechanism of free fatty acid re-esterification in human adipocytes in vitro. J Lipid Res. 1990;31(8):1423–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

