Intestinal Microbiome-Macrophage Crosstalk Contributes to Cholestatic Liver Disease by Promoting Intestinal Permeability in Mice
- PMID: 32168395
- PMCID: PMC7839474
- DOI: 10.1002/hep.31228
Intestinal Microbiome-Macrophage Crosstalk Contributes to Cholestatic Liver Disease by Promoting Intestinal Permeability in Mice
Abstract
Background and aims: Mounting evidence supports an association between cholestatic liver disease and changes in the composition of the microbiome. Still, the role of the microbiome in the pathogenesis of this condition remains largely undefined.
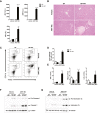
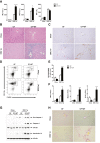
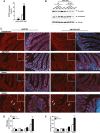
Approach and results: To address this, we have used two experimental models, administering alpha-naphtylisocyanate or feeding a 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet, to induce cholestatic liver disease in germ-free mice and germ-free mice conventionalized with the microbiome from wild-type, specific pathogen-free animals. Next, we have inhibited macrophage activation by depleting these cells using clodronate liposomes and inhibiting the inflammasome with a specific inhibitor of NOD-, LRR-, and pyrin domain-containing protein 3. Our results demonstrate that cholestasis, the accumulation of bile acids in the liver, fails to promote liver injury in the absence of the microbiome in vivo. Additional in vitro studies supported that endotoxin sensitizes hepatocytes to bile-acid-induced cell death. We also demonstrate that during cholestasis, macrophages contribute to promoting intestinal permeability and to altered microbiome composition through activation of the inflammasome, overall leading to increased endotoxin flux into the cholestatic liver.
Conclusions: We demonstrate that the intestinal microbiome contributes to cholestasis-mediated cell death and inflammation through mechanisms involving activation of the inflammasome in macrophages.
© 2020 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- BB/R012512/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/CCG1860/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004529/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10347/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

