Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer's Disease Treatment
- PMID: 32168767
- PMCID: PMC7151011
- DOI: 10.3390/pharmaceutics12030251
Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer's Disease Treatment
Abstract
Direct nose-to-brain delivery has been raised as a non-invasive powerful strategy to deliver drugs to the brain bypassing the blood-brain barrier (BBB). This study aimed at preparing and characterizing an innovative composite formulation, associating the liposome and hydrogel approaches, suitable for intranasal administration. Thermosensitive gel formulations were obtained based on a mixture of two hydrophilic polymers (Poloxamer 407, P407 and Poloxamer 188, P188) for a controlled delivery through nasal route via liposomes of an active pharmaceutical ingredient (API) of potential interest for Alzheimer's disease. The osmolarity and the gelation temperature (T° sol-gel) of formulations, defined in a ternary diagram, were investigated by rheometry and visual determination. Regarding the issue of assays, a mixture composed of P407/P188 (15/1%, w/w) was selected for intranasal administration in terms of T° sol-gel and for the compatibility with the olfactory mucosal (280 ± 20 mOsmol, pH 6). Liposomes of API were prepared by the thin film hydration method. Mucoadhesion studies were performed by using mucin disc, and they showed the good natural mucoadhesive characteristics of in situ gel formulations, which increased when liposomes were added. The study demonstrated successful pharmacotechnical development of a promising API-loaded liposomes in a thermosensitive hydrogel intended for nasal Alzheimer's disease treatment.
Keywords: nanocomposite; nose-to-brain delivery; thermosensitive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 low T°sol-gel transition,
low T°sol-gel transition,  acceptable T°sol-gel transition,
acceptable T°sol-gel transition,  high T°sol-gel transition; (b):
high T°sol-gel transition; (b):  acceptable osmolarity,
acceptable osmolarity,  High osmolarity.
High osmolarity.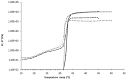

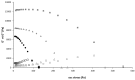

 detachment Force (mN),
detachment Force (mN),  adhesion work (mN.mm).
adhesion work (mN.mm).
 Solution API 1 mg/mL,
Solution API 1 mg/mL,  API-loaded liposomes ,
API-loaded liposomes ,  Gel with API 1mg/mL,
Gel with API 1mg/mL,  API-loaded Liposomes in gel.
API-loaded Liposomes in gel.References
-
- Hoffmann M., Stiller C., Endres E., Scheiner M., Gunesch S., Sotriffer C., Maurice T., Decker M. Highly Selective Butyrylcholinesterase Inhibitors with Tunable Duration of Action by Chemical Modification of Transferable Carbamate Units Exhibit Pronounced Neuroprotective Effect in an Alzheimer’s Disease Mouse Model. J. Med. Chem. 2019;62:9116–9140. doi: 10.1021/acs.jmedchem.9b01012. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

