Resolution of alkaloid racemate: a novel microbial approach for the production of enantiopure lupanine via industrial wastewater valorization
- PMID: 32169079
- PMCID: PMC7071741
- DOI: 10.1186/s12934-020-01324-1
Resolution of alkaloid racemate: a novel microbial approach for the production of enantiopure lupanine via industrial wastewater valorization
Abstract
Background: Lupanine is a plant toxin contained in the wastewater of lupine bean processing industries, which could be used for semi-synthesis of various novel high added-value compounds. This paper introduces an environmental friendly process for microbial production of enantiopure lupanine.
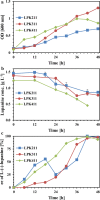
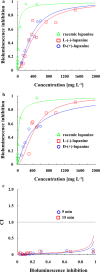
Results: Previously isolated P. putida LPK411, R. rhodochrous LPK211 and Rhodococcus sp. LPK311, holding the capacity to utilize lupanine as single carbon source, were employed as biocatalysts for resolution of racemic lupanine. All strains achieved high enantiomeric excess (ee) of L-(-)-lupanine (> 95%), while with the use of LPK411 53% of the initial racemate content was not removed. LPK411 fed with lupanine enantiomers as single substrates achieved 92% of D-(+)-lupanine biodegradation, whereas L-(-)-lupanine was not metabolized. Monitoring the transcriptional kinetics of the luh gene in cultures supplemented with the racemate as well as each of the enantiomers supported the enantioselectivity of LPK411 for D-(+)-lupanine biotransformation, while (trans)-6-oxooctahydro-1H-quinolizine-3-carboxylic acid was detected as final biodegradation product from D-(+)-lupanine use. Ecotoxicological assessment demonstrated that lupanine enantiomers were less toxic to A. fischeri compared to the racemate exhibiting synergistic interaction.
Conclusions: The biological chiral separation process of lupanine presented here constitutes an eco-friendly and low-cost alternative to widely used chemical methods for chiral separation.
Keywords: Ecotoxicological assessment; Enantiomeric excess; Enantiomers; Gene expression; Lupanine; Pseudomonas putida LPK411; Quantitative real-time PCR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Blaser HU. Looking back on 35 years of industrial catalysis. Chimia. 2015;69(7–8):393–406. - PubMed
-
- Calcaterra A, D’Acquarica I. The market of chiral drugs: chiral switches versus de novo enantiomerically pure compounds. J Pharm Biomed Anal. 2018;147:323–340. - PubMed
-
- Sanganyado E, Lu Z, Fu Q, Schlenk D, Gan J. Chiral pharmaceuticals: a review on their environmental occurrence and fate processes. Water Res. 2017;124:527–542. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

