Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings
- PMID: 32169115
- PMCID: PMC7071711
- DOI: 10.1186/s40644-020-00300-7
Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings
Abstract
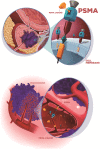
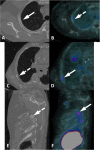
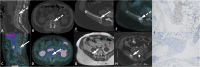
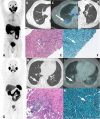
PSMA PET imaging was originally used to assess biochemical recurrence of prostate cancer (PCa), but its clinical use was promptly extended to detection, staging and therapy response assessment. The expanding use of PSMA PET worldwide has also revealed PSMA ligand uptake in diverse nonprostatic diseases, which raised questions about the specificity of this imaging modality. Although not very common initially, a growing number of pathologies presenting PSMA uptake on PET have been reported in the last few years, and a proper interpretation of PSMA PET imaging findings suddenly became challenging and, to some extent, confusing. Compared to cytoplasmic PSMA expression in nonprostatic cells, the molecular features of apical PSMA expression in PCa cells can help to distinguish these various conditions. Correlations of imaging findings to patient history, to the expected pattern of disease spread and mainly to computed tomography (CT) and/or magnetic resonance imaging (MRI) characteristics will reinforce the distinction of lesions that are more likely related to PCa from those that could lead to an incorrect diagnosis. The overall benefits of endothelial PSMA expression, which is associated with the neovasculature of malignant neoplasms, will be highlighted, stating the potential use of PSMA ligand uptake as a theranostic tool. This review aims to cover the collection of nonprostatic diseases, including benign and malignant tumors, in a didactic approach according to disease etiology, with discussion of bone-related conditions and inflammatory and infectious processes.
Keywords: (68)Ga-PSMA; Positron emission tomography; Prostate cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








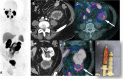
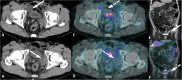






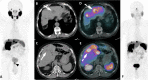

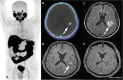
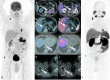
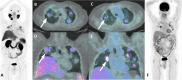
References
-
- Perera Marlon, Papa Nathan, Roberts Matthew, Williams Michael, Udovicich Cristian, Vela Ian, Christidis Daniel, Bolton Damien, Hofman Michael S., Lawrentschuk Nathan, Murphy Declan G. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. European Urology. 2020;77(4):403–417. doi: 10.1016/j.eururo.2019.01.049. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

