Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report
- PMID: 32169158
- PMCID: PMC7606918
- DOI: 10.1016/S2352-3018(20)30069-2
Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report
Abstract
Background: The London patient (participant 36 in the IciStem cohort) underwent allogeneic stem-cell transplantation with cells that did not express CCR5 (CCR5Δ32/Δ32); remission was reported at 18 months after analytical treatment interruption (ATI). Here, we present longer term data for this patient (up to 30 months after ATI), including sampling from diverse HIV-1 reservoir sites.
Methods: We used ultrasensitive viral load assays of plasma, semen, and cerebrospinal fluid (CSF) samples to detect HIV-1 RNA. In gut biopsy samples and lymph-node tissue, cell-copy number and total HIV-1 DNA levels were quantified in multiple replicates, using droplet digital PCR (ddPCR) and quantitative real-time PCR. We also analysed the presence of intact proviral DNA using multiplex ddPCR targeting the packaging signal (ψ) and envelope (env). We did intracellular cytokine staining to measure HIV-1-specific T-cell responses. We used low-sensitive and low-avidity antibody assays to measure the humoral response to HIV-1. We predicted the probability of rebound using a mathematical model and inference approach.
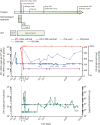
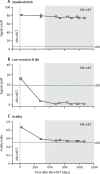
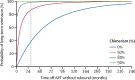
Findings: HIV-1 viral load in plasma remained undetectable in the London patient up to 30 months (last tested on March 4, 2020), using an assay with a detection limit of 1 copy per mL. The patient's CD4 count was 430 cells per μL (23·5% of total T cells) at 28 months. A very low-level positive signal for HIV-1 DNA was recorded in peripheral CD4 memory cells at 28 months. The viral load in semen was undetectable in both plasma (lower limit of detection [LLD] <12 copies per mL) and cells (LLD 10 copies per 106 cells) at 21 months. CSF was within normal parameters at 25 months, with HIV-1 RNA below the detection limit (LLD 1 copy per mL). HIV-1 DNA by ddPCR was negative in rectum, caecum, and sigmoid colon and terminal ileum tissue samples at 22 months. Lymph-node tissue from axilla was positive for the long-terminal repeat (33 copies per 106 cells) and env (26·1 copies per 106 cells), negative for ψ and integrase, and negative by the intact proviral DNA assay, at 27 months. HIV-1-specific CD4 and CD8 T-cell responses have remained absent at 27 months. Low-avidity Env antibodies have continued to decline. Mathematical modelling suggests that the probability of remission for life (cure) is 98% in the context of 80% donor chimerism in total HIV target cells and greater than 99% probability of remission for life with 90% donor chimerism.
Interpretation: The London patient has been in HIV-1 remission for 30 months with no detectable replication-competent virus in blood, CSF, intestinal tissue, or lymphoid tissue. Donor chimerism has been maintained at 99% in peripheral T cells. We propose that these findings represent HIV-1 cure.
Funding: Wellcome Trust and amfAR (American Foundation for AIDS Research).
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
A cure for HIV: how would we know?Lancet HIV. 2020 May;7(5):e304-e306. doi: 10.1016/S2352-3018(20)30075-8. Epub 2020 Mar 10. Lancet HIV. 2020. PMID: 32169159 No abstract available.
References
-
- Hutter G, Nowak D, Mossner M. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. - PubMed
-
- Hutter G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: an update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–274. - PubMed
-
- Allers K, Hutter G, Hofmann J. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
-
- Thornhill JP, Pace M, Martin GE. CD32 expressing doublets in HIV-infected gut-associated lymphoid tissue are associated with a T follicular helper cell phenotype. Mucosal Immunol. 2019;12:1212–1219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

