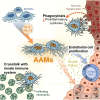
Alternatively activated macrophages promote resolution of necrosis following acute liver injury
- PMID: 32169610
- PMCID: PMC7378576
- DOI: 10.1016/j.jhep.2020.02.031
Alternatively activated macrophages promote resolution of necrosis following acute liver injury
Abstract
Background & aim: Following acetaminophen (APAP) overdose, acute liver injury (ALI) can occur in patients that present too late for N-acetylcysteine treatment, potentially leading to acute liver failure, systemic inflammation, and death. Macrophages influence the progression and resolution of ALI due to their innate immunological function and paracrine activity. Syngeneic primary bone marrow-derived macrophages (BMDMs) were tested as a cell-based therapy in a mouse model of APAP-induced ALI (APAP-ALI).
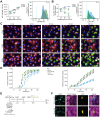
Methods: Several phenotypically distinct BMDM populations were delivered intravenously to APAP-ALI mice when hepatic necrosis was established, and then evaluated based on their effects on injury, inflammation, immunity, and regeneration. In vivo phagocytosis assays were used to interrogate the phenotype and function of alternatively activated BMDMs (AAMs) post-injection. Finally, primary human AAMs sourced from healthy volunteers were evaluated in immunocompetent APAP-ALI mice.
Results: BMDMs rapidly localised to the liver and spleen within 4 h of administration. Injection of AAMs specifically reduced hepatocellular necrosis, HMGB1 translocation, and infiltrating neutrophils following APAP-ALI. AAM delivery also stimulated proliferation in hepatocytes and endothelium, and reduced levels of several circulating proinflammatory cytokines within 24 h. AAMs displayed a high phagocytic activity both in vitro and in injured liver tissue post-injection. Crosstalk with the host innate immune system was demonstrated by reduced infiltrating host Ly6Chi macrophages in AAM-treated mice. Importantly, therapeutic efficacy was partially recapitulated using clinical-grade primary human AAMs in immunocompetent APAP-ALI mice, underscoring the translational potential of these findings.
Conclusion: We identify that AAMs have value as a cell-based therapy in an experimental model of APAP-ALI. Human AAMs warrant further evaluation as a potential cell-based therapy for APAP overdose patients with established liver injury.
Lay summary: After an overdose of acetaminophen (paracetamol), some patients present to hospital too late for the current antidote (N-acetylcysteine) to be effective. We tested whether macrophages, an injury-responsive leukocyte that can scavenge dead/dying cells, could serve as a cell-based therapy in an experimental model of acetaminophen overdose. Injection of alternatively activated macrophages rapidly reduced liver injury and reduced several mediators of inflammation. Macrophages show promise to serve as a potential cell-based therapy for acute liver injury.
Keywords: Acetaminophen; Liver regeneration; Macrophages; Necrosis; Phagocytosis.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest P.S.L., L.F., and S.J.F. have patents pending, entitled ‘Macrophage-based therapy’ in national territories of USA, Europe, Japan, China and Australia. These patents have been derived from PCT/GB2017/052769 filed 18/09/2017 and claim priority from UK application 1615923.8 filed 19/09/2016. Both of the original patents have now been abandoned because the original UK patent and PCT patent are no longer live and have now been replaced by the national patents. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Larson A.M., Polson J., Fontana R.J., Davern T.J., Lalani E., Hynan L.S. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364. - PubMed
-
- Lee W.M. Acetaminophen and the U.S. acute liver failure study group: lowering the risks of hepatic failure. Hepatology. 2004;40:6. - PubMed
-
- Lee W.M. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38:S3. - PubMed
-
- Rolando N., Wade J., Davalos M., Wendon J., Philpott-Howard J., Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

