Hydrolyzed Ce(IV) salts limit sucrose-dependent biofilm formation by Streptococcus mutans
- PMID: 32169780
- PMCID: PMC7233464
- DOI: 10.1016/j.jinorgbio.2020.110997
Hydrolyzed Ce(IV) salts limit sucrose-dependent biofilm formation by Streptococcus mutans
Abstract
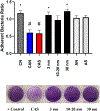
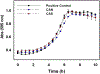
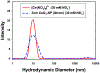
Several studies have focused on the antimicrobial effects of cerium oxide nanoparticles (CeO2-NP) but few have focused on their effects on bacteria under initial biofilm formation conditions. Streptococcus mutans is a prolific biofilm former contributing to dental caries in the presence of fermentable carbohydrates and is a recognized target for therapeutic intervention. CeO2-NP derived solely from Ce(IV) salt hydrolysis were found to reduce adherent bacteria by approximately 40% while commercial dispersions of "bare" CeO2-NP (e.g., 3 nm, 10-20 nm, 30 nm diameter) and Ce(NO3)3·6H2O were either inactive or observed to slightly increase biofilm formation under similar in vitro conditions. Planktonic growth and dispersal assays support a non-bactericidal mode of biofilm inhibition active in the initial phases of S. mutans biofilm production. Human cell proliferation assays suggest only minor effects of hydrolyzed Ce(IV) salts on cellular metabolism at concentrations up to 1 mM Ce, with less observed toxicity compared to equimolar concentrations of AgNO3. The results presented herein have implications in clinical dentistry.
Keywords: Biofilm; Ce(IV); Dental; Inhibition; Streptococcus mutans.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest We report no conflicts of interest in relation to this study or the materials utilized.
Figures

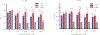
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

