Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes
- PMID: 32170044
- PMCID: PMC7136063
- DOI: 10.1212/NXI.0000000000000701
Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes
Abstract
Objective: To investigate the diagnostic yield of commercial immunodots to detect onconeural antibodies associated with paraneoplastic neurologic syndromes (PNSs), we analyzed the proportion of confirmed positive results using alternative techniques.
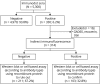
Methods: Sera (n = 5,300) of patients with suspected PNS were tested by PNS+2 blot (Ravo Diagnostika; January 2016-May 2017) or EUROLINE PNS 12 Ag (Euroimmun; July 2017-November 2018). Positive samples were further explored by in-house indirect immunofluorescence and a third in-house technique (Western blot or cell-based assay) using recombinant protein. Those found negative by these 2 techniques were considered as nonconfirmed. We analyzed the relationship between band intensity and final confirmation. Clinical data were collected for all confirmed results and nonconfirmed EUROLINE immunodots.
Results: PNS+2 blot was positive in 128/1,658 (7.7%) sera and confirmed in 47/128 (36.7%). EUROLINE was positive in 186/3,626 (5.1%) and confirmed in 56/186 (30.1%). Confirmation was highly variable among the antibodies tested, from 7.2% (PNS+2 blot) and 5.8% (EUROLINE) for anti-Yo to 88.2% (PNS+2 blot) and 65.0% (EUROLINE) for anti-Hu. None of the 27 weak positive sera by EUROLINE was confirmed. Band intensity in confirmed cases was variable among the antibodies from strong positive for all anti-Yo (n = 3) and anti-Hu (n = 11) to positive (n = 19) or strong positive (n = 9) for anti-SOX1. Among patients with a nonconfirmed EUROLINE result and available clinical information, all had an alternative diagnosis, and only 6.7% had cancer.
Conclusions: Immunodots may be useful for PNS screening, but a threshold should be established for each antibody, and clinical information and confirmation by other techniques are essential.
Classification of evidence: The study provides Class IV evidence that immunodot assays for onconeural antibodies accurately identify patients with paraneoplastic neurologic syndromes.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
