Long-term efficacy of mycophenolate mofetil in myelin oligodendrocyte glycoprotein antibody-associated disorders: A prospective study
- PMID: 32170045
- PMCID: PMC7136046
- DOI: 10.1212/NXI.0000000000000705
Long-term efficacy of mycophenolate mofetil in myelin oligodendrocyte glycoprotein antibody-associated disorders: A prospective study
Abstract
Objective: To investigate whether the use of mycophenolate mofetil (MMF) could reduce the relapse risk in patients with myelin oligodendrocyte glycoprotein (MOG)-immunoglobulin G (IgG)-associated disorders (MOGADs).
Methods: This prospective observational cohort study included patients with MOGAD at Peking Union Medical College Hospital between January 1, 2017, and April 30, 2019. The patients were divided into 2 groups: those with (MMF+) or without (MMF-) MMF therapy. The primary outcome was relapse at follow-up. We used Cox proportional hazards models to calculate hazard ratios (HRs) for relapse.
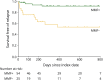
Results: Seventy-nine patients were included in our MOG cohort. Fifty (63.3%) were adults at index date, and 47 (59.5%) were women. Fifty-four (68.4%) were in the MMF+ group, and 25 (31.6%) were in the MMF- group. Clinical and demographic factors, MOG-IgG titer, and follow-up time (median, 472.5 days for MMF+, 261.0 days for MMF-) were comparable between the groups. Relapse rates were 7.4% (4/54) in the MMF+ group and 44.0% (11/25) in the MMF- group. Of all potential confounders, only the use of MMF was associated with reduced risk of relapse. The HR for relapse among patients in the MMF+ group was 0.14 (95% CI, 0.05-0.45) and was 0.08 (95% CI, 0.02-0.28) in a model adjusted for age, sex, disease course, and MOG-IgG titer. MMF therapy also remained associated with a reduced relapse risk in sensitivity analyses. Only one patient (1.9%) discontinued MMF therapy because of adverse effect.
Conclusions: These findings provide a clinical evidence that MMF immunosuppression therapy may prevent relapse in patients with MOGAD.
Classification of evidence: This study provides class IV evidence that for patients with MOGAD, MMF reduces relapse risk.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Reindl M, Di Pauli F, Rostasy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 2013;9:455–461. - PubMed
-
- Waters P, Woodhall M, O'Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89 doi: 10.1212/NXI.0000000000000089. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
