Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors
- PMID: 32170060
- PMCID: PMC7070094
- DOI: 10.1038/s41467-020-15230-y
Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors
Abstract
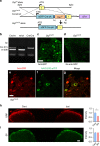
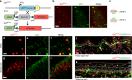
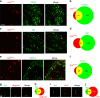
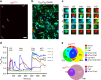
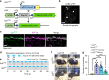
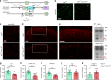
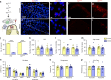
Gastrin-releasing peptide (GRP) functions as a neurotransmitter for non-histaminergic itch, but its site of action (sensory neurons vs spinal cord) remains controversial. To determine the role of GRP in sensory neurons, we generated a floxed Grp mouse line. We found that conditional knockout of Grp in sensory neurons results in attenuated non-histaminergic itch, without impairing histamine-induced itch. Using a Grp-Cre knock-in mouse line, we show that the upper epidermis of the skin is exclusively innervated by GRP fibers, whose activation via optogeneics and chemogenetics in the skin evokes itch- but not pain-related scratching or wiping behaviors. In contrast, intersectional genetic ablation of spinal Grp neurons does not affect itch nor pain transmission, demonstrating that spinal Grp neurons are dispensable for itch transmission. These data indicate that GRP is a neuropeptide in sensory neurons for non-histaminergic itch, and GRP sensory neurons are dedicated to itch transmission.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

