Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping
- PMID: 32170079
- PMCID: PMC7070030
- DOI: 10.1038/s41467-020-14957-y
Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping
Abstract
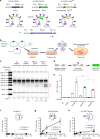
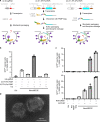
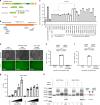
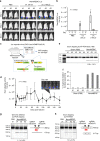
Prolonged expression of the CRISPR-Cas9 nuclease and gRNA from viral vectors may cause off-target mutagenesis and immunogenicity. Thus, a transient delivery system is needed for therapeutic genome editing applications. Here, we develop an extracellular nanovesicle-based ribonucleoprotein delivery system named NanoMEDIC by utilizing two distinct homing mechanisms. Chemical induced dimerization recruits Cas9 protein into extracellular nanovesicles, and then a viral RNA packaging signal and two self-cleaving riboswitches tether and release sgRNA into nanovesicles. We demonstrate efficient genome editing in various hard-to-transfect cell types, including human induced pluripotent stem (iPS) cells, neurons, and myoblasts. NanoMEDIC also achieves over 90% exon skipping efficiencies in skeletal muscle cells derived from Duchenne muscular dystrophy (DMD) patient iPS cells. Finally, single intramuscular injection of NanoMEDIC induces permanent genomic exon skipping in a luciferase reporter mouse and in mdx mice, indicating its utility for in vivo genome editing therapy of DMD and beyond.
Conflict of interest statement
P.G. and A.H. are inventors on a filed patent (PCT/JP2019/027708) based on the delivery system, virus-like particle, production, and use for genome editing described here. Y.M., H.H., and N.I. are employees of Takeda Pharmaceutical Company. A.H. is a principle investigator (without salary) of the T-CiRA program funded by Takeda Pharmaceutical Company. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

