Establishment, optimisation and quantitation of a bioluminescent murine infection model of visceral leishmaniasis for systematic vaccine screening
- PMID: 32170135
- PMCID: PMC7070049
- DOI: 10.1038/s41598-020-61662-3
Establishment, optimisation and quantitation of a bioluminescent murine infection model of visceral leishmaniasis for systematic vaccine screening
Erratum in
-
Author Correction: Establishment, optimisation and quantitation of a bioluminescent murine infection model of visceral leishmaniasis for systematic vaccine screening.Sci Rep. 2021 Apr 7;11(1):8015. doi: 10.1038/s41598-021-87190-2. Sci Rep. 2021. PMID: 33828148 Free PMC article. No abstract available.
Abstract
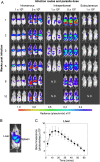
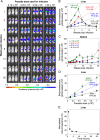
Visceral leishmaniasis is an infectious parasitic disease caused by the protozoan parasites Leishmania donovani and Leishmania infantum. The drugs currently used to treat visceral leishmaniasis suffer from toxicity and the emergence of parasite resistance, and so a better solution would be the development of an effective subunit vaccine; however, no approved vaccine currently exists. The comparative testing of a large number of vaccine candidates requires a quantitative and reproducible experimental murine infection model, but the parameters that influence infection pathology have not been systematically determined. To address this, we have established an infection model using a transgenic luciferase-expressing L. donovani parasite and longitudinally quantified the infections using in vivo bioluminescent imaging within individual mice. We examined the effects of varying the infection route, the site of adjuvant formulation administration, and standardised the parasite preparation and dose. We observed that the increase in parasite load within the liver during the first few weeks of infection was directly proportional to the parasite number in the initial inoculum. Finally, we show that immunity can be induced in pre-exposed animals that have resolved an initial infection. This murine infection model provides a platform for systematic subunit vaccine testing against visceral leishmaniasis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. Leishmaniasis. https://wwwwhoint/news-room/fact-sheets/detail/leishmaniasis. (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

