Antibiotic Treatments During Infancy, Changes in Nasal Microbiota, and Asthma Development: Population-based Cohort Study
- PMID: 32170305
- PMCID: PMC8096219
- DOI: 10.1093/cid/ciaa262
Antibiotic Treatments During Infancy, Changes in Nasal Microbiota, and Asthma Development: Population-based Cohort Study
Abstract
Background: Early-life exposures to antibiotics may increase the risk of developing childhood asthma. However, little is known about the mechanisms linking antibiotic exposures to asthma. We hypothesized that changes in the nasal airway microbiota serve as a causal mediator in the antibiotics-asthma link.
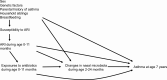
Methods: In a population-based birth-cohort study in Finland, we identified longitudinal nasal microbiota profiles during age 2-24 months using 16S rRNA gene sequencing and an unsupervised machine learning approach. We performed a causal mediation analysis to estimate the natural direct effect of systemic antibiotic treatments during age 0-11 months on risks of developing physician-diagnosed asthma by age 7 years and the natural indirect (causal mediation) effect through longitudinal changes in nasal microbiota.
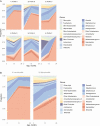
Results: In our birth cohort of 697 children, 8.0% later developed asthma. Exposure to ≥2 antibiotic treatments during age 0-11 months was associated with a 4.0% increase in the absolute risk of developing asthma (absolute increase, 95% CI, .9-7.2%; P = .006). The unsupervised clustering approach identified 6 longitudinal nasal microbiota profiles. Infants with a larger number of antibiotic treatments had a higher risk of having a profile with early Moraxella sparsity (per each antibiotic treatment, adjusted RRR, 1.38; 95% CI, 1.15-1.66; P < .001). This effect of antibiotics on asthma was partly mediated by longitudinal changes in the nasal microbiota (natural indirect effect, P = .008), accounting for 16% of the total effect.
Conclusions: Early exposures to antibiotics were associated with increased risk of asthma; the effect was mediated, in part, by longitudinal changes in the nasal airway microbiota.
Keywords: airway microbiota; antibiotics; asthma; causal mediation; children.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
New Insights Into the Role of Antibiotic Use in Infancy and the Upper Airway Microbiome in Childhood Asthma Development.Clin Infect Dis. 2021 May 4;72(9):1555-1556. doi: 10.1093/cid/ciaa266. Clin Infect Dis. 2021. PMID: 32170322 Free PMC article. No abstract available.
Similar articles
-
Longitudinal Changes in Early Nasal Microbiota and the Risk of Childhood Asthma.Pediatrics. 2020 Oct;146(4):e20200421. doi: 10.1542/peds.2020-0421. Epub 2020 Sep 15. Pediatrics. 2020. PMID: 32934151
-
Maturation of nasal microbiota and antibiotic exposures during early childhood: a population-based cohort study.Clin Microbiol Infect. 2021 Feb;27(2):283.e1-283.e7. doi: 10.1016/j.cmi.2020.05.033. Epub 2020 Jun 4. Clin Microbiol Infect. 2021. PMID: 32505584
-
Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies.Lancet Respir Med. 2020 Nov;8(11):1094-1105. doi: 10.1016/S2213-2600(20)30052-7. Epub 2020 Mar 24. Lancet Respir Med. 2020. PMID: 32220282
-
Lessons learned from birth cohort studies conducted in diverse environments.J Allergy Clin Immunol. 2017 Feb;139(2):379-386. doi: 10.1016/j.jaci.2016.12.941. J Allergy Clin Immunol. 2017. PMID: 28183432 Free PMC article. Review.
-
Use of Antibiotics in Infancy and Asthma in Childhood: Confounded or Causal Relationship? A Critical Review of the Literature.J Allergy Clin Immunol Pract. 2024 Oct;12(10):2669-2677. doi: 10.1016/j.jaip.2024.06.018. Epub 2024 Jun 18. J Allergy Clin Immunol Pract. 2024. PMID: 38901616 Review.
Cited by
-
Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response.Front Immunol. 2022 Sep 30;13:1028209. doi: 10.3389/fimmu.2022.1028209. eCollection 2022. Front Immunol. 2022. PMID: 36248891 Free PMC article. Review.
-
Risk of infantile atopic dermatitis in neonatal lupus erythematosus: a retrospective cohort study.Front Immunol. 2025 Mar 27;16:1517687. doi: 10.3389/fimmu.2025.1517687. eCollection 2025. Front Immunol. 2025. PMID: 40213542 Free PMC article.
-
Winds of change a tale of: asthma and microbiome.Front Microbiol. 2023 Dec 11;14:1295215. doi: 10.3389/fmicb.2023.1295215. eCollection 2023. Front Microbiol. 2023. PMID: 38146448 Free PMC article. Review.
-
Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma.Nat Commun. 2021 Jun 14;12(1):3601. doi: 10.1038/s41467-021-23859-6. Nat Commun. 2021. PMID: 34127671 Free PMC article.
-
New Insights Into the Role of Antibiotic Use in Infancy and the Upper Airway Microbiome in Childhood Asthma Development.Clin Infect Dis. 2021 May 4;72(9):1555-1556. doi: 10.1093/cid/ciaa266. Clin Infect Dis. 2021. PMID: 32170322 Free PMC article. No abstract available.
References
-
- Moorman JE, Akinbami LJ, Bailey CM, et al. . National surveillance of asthma: United States, 2001–2010. Vital Health Stat 2012; 3:1–58. - PubMed
-
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S; International Study of Asthma and Allergies in Childhood Phase Three Study Group . Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009; 64:476–83. - PubMed
-
- van de Maat J, van de Voort E, Mintegi S, et al. . Antibiotic prescription for febrile children in European emergency departments: a cross-sectional, observational study. Lancet Infect Dis 2019; 19:382–91. - PubMed

