Multispectral singlet oxygen and photosensitizer luminescence dosimeter for continuous photodynamic therapy dose assessment during treatment
- PMID: 32170859
- PMCID: PMC7068220
- DOI: 10.1117/1.JBO.25.6.063810
Multispectral singlet oxygen and photosensitizer luminescence dosimeter for continuous photodynamic therapy dose assessment during treatment
Abstract
Significance: Photodynamic therapy (PDT) involves complex light-drug-pathophysiology interactions that can be affected by multiple parameters and often leads to large variations in treatment outcome from patient to patient. Direct PDT dosimetry technologies have been sought to optimize the control variables (e.g., light dose, drug administration, tissue oxygenation, and patient conditioning) for best patient outcomes. In comparison, singlet oxygen (O21) dosimetry has been tested in various forms to provide an accurate and perhaps comprehensive prediction of the treatment efficacy.
Aim: We discuss an advanced version of this approach provided by a noninvasive, continuous wave dosimeter that can measure near-infrared spectrally resolved luminescence of both photosensitizer (PS) and O21 generated during PDT cancer treatment.
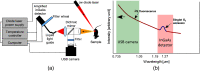
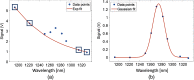
Approach: This dosimetry technology uses an amplified, high quantum efficiency InGaAs detector with spectroscopic decomposition during the light treatment to continuously extract the maximum signal of O21 phosphorescence while suppressing the strong PS luminescence background by spectrally fitting the data points across nine narrow band wavelengths. O21 and PS luminescence signals were measured in vivo in FaDu xenograft tumors grown in mice during PDT treatment using Verteporfin as the PS and a continuous laser treatment at 690 nm wavelength.
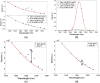
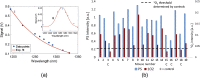
Results: A cohort of 19 mice was used and observations indicate that the tumor growth rate inhibition showed a stronger correlation with O21 than with just the PS signal.
Conclusions: These results suggest that O21 measurement may be a more direct dosimeter of PDT damage, and it has potential value as a definitive diagnostic for PDT treatment, especially with spectral separation of the background luminescence and online estimation of the PS concentration.
Keywords: dose; photodynamic therapy; singlet oxygen; spectroscopy; tumor regrowth.
Figures



References
-
- Vrouenraets M. B., et al. , “Basic principles, applications in oncology and improved selectivity of photodynamic therapy,” Anticancer Res. 23(1b), 505–522 (2003). - PubMed

