Sonographic and 3T-MRI-based evaluation of the tongue in ALS
- PMID: 32171167
- PMCID: PMC7068685
- DOI: 10.1016/j.nicl.2020.102233
Sonographic and 3T-MRI-based evaluation of the tongue in ALS
Abstract
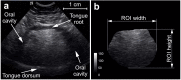
A few systematic imaging studies employing ultrasound (HRUS) and magnetic resonance imaging (MRI) have suggested tongue measures to aid in diagnosis of amyotrophic lateral sclerosis (ALS). The relationship between structural tongue alterations and the ALS patients' bulbar and overall motor function has not yet been elucidated. We here thus aimed to understand how in-vivo tongue alterations relate to motor function and motor function evolution over time in ALS. Our study included 206 ALS patients and 104 age- and sex-matched controls that underwent HRUS and 3T MRI of the tongue at baseline. Sonographic measures comprised coronal tongue echointensity, area, height, width and height/width ratio, while MRI measures comprised sagittal T1 intensity, tongue area, position and shape. Imaging-derived markers were related to baseline and longitudinal bulbar and overall motor function. Baseline T1 intensity was lower in ALS patients with more severe bulbar involvement at baseline. Smaller baseline coronal (HRUS) and sagittal (MRI) tongue area, smaller coronal height (HRUS) and width (HRUS) as well as more rounded sagittal tongue shape predicated more rapid functional impairment - not only of bulbar, but also of overall motor function - in ALS. Our results suggest that in-vivo sonography und MRI tongue measures could aid as biomarkers to reflect bulbar and motor function impairment.
Keywords: Amyotrophic lateral sclerosis; Biomarker; Intensity; MRI; Prognosis; Tongue; Ultrasound.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests None of the authors have potential conflicts of interest to be disclosed.
Figures




References
-
- Ahmadi M., Liu J.-.X., Brännström T., Andersen P.M., Stål P., Pedrosa-Domellöf F. Human extraocular muscles in ALS. Invest. Ophthalmol. Vis. Sci. 2010;51(7):3494–3501. - PubMed
-
- Al Chalabi A., Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013;9(11):617–628. - PubMed
-
- Al Chalabi A., Hardiman O., Kiernan M.C., Chio A., Rix-Brooks B., van den Berg L.H. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016;15(11):1182–1194. - PubMed
-
- Al-Sarraj S., King A., Cleveland M., Pradat P.-.F., Corse A., Rothstein J.D., Leigh P.N., Abila B., Bates S., Wurthner J., Meininger V. Mitochondrial abnormalities and low grade inflammation are present in the skeletal muscle of a minority of patients with amyotrophic lateral sclerosis; an observational myopathology study. Acta Neuropathol. Commun. 2014;2:165. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

