Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration
- PMID: 32171310
- PMCID: PMC7073016
- DOI: 10.1186/s12929-020-0624-8
Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration
Abstract
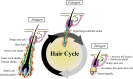
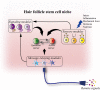
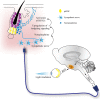
Stem cell activity is subject to non-cell-autonomous regulation from the local microenvironment, or niche. In adaption to varying physiological conditions and the ever-changing external environment, the stem cell niche has evolved with multifunctionality that enables stem cells to detect these changes and to communicate with remote cells/tissues to tailor their activity for organismal needs. The cyclic growth of hair follicles is powered by hair follicle stem cells (HFSCs). Using HFSCs as a model, we categorize niche cells into 3 functional modules, including signaling, sensing and message-relaying. Signaling modules, such as dermal papilla cells, immune cells and adipocytes, regulate HFSC activity through short-range cell-cell contact or paracrine effects. Macrophages capacitate the HFSC niche to sense tissue injury and mechanical cues and adipocytes seem to modulate HFSC activity in response to systemic nutritional states. Sympathetic nerves implement the message-relaying function by transmitting external light signals through an ipRGC-SCN-sympathetic circuit to facilitate hair regeneration. Hair growth can be disrupted by niche pathology, e.g. dysfunction of dermal papilla cells in androgenetic alopecia and influx of auto-reacting T cells in alopecia areata and lichen planopilaris. Understanding the functions and pathological changes of the HFSC niche can provide new insight for the treatment of hair loss.
Keywords: Alopecia; Alopecia areata; Androgenetic alopecia; Function; Hair follicle stem cell; Lichen planopilaris; Niche; Therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

