Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study
- PMID: 32171872
- PMCID: PMC7156152
- DOI: 10.1016/j.jinf.2020.03.002
Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study
Abstract
Background: Corona Virus Disease 2019 (COVID-19) due to the 2019 novel coronavirus (SARS-CoV-2) emerged in Wuhan city and rapidly spread throughout China. We aimed to compare arbidol and lopinavir/ritonavir(LPV/r) treatment for patients with COVID-19 with LPV/r only.
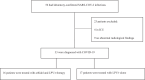
Methods: In this retrospective cohort study, we included adults (age≥18years) with laboratory-confirmed COVID-19 without Invasive ventilation, diagnosed between Jan 17, 2020, and Feb 13, 2020. Patients, diagnosed after Jan 17, 2020, were given oral arbidol and LPV/r in the combination group and oral LPV/r only in the monotherapy group for 5-21 days. The primary endpoint was a negative conversion rate of coronavirus from the date of COVID-19 diagnosis(day7, day14), and assessed whether the pneumonia was progressing or improving by chest CT (day7).
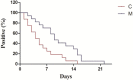
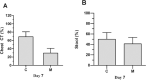
Results: We analyzed 16 patients who received oral arbidol and LPV/r in the combination group and 17 who oral LPV/r only in the monotherapy group, and both initiated after diagnosis. Baseline clinical, laboratory, and chest CT characteristics were similar between groups. The SARS-CoV-2 could not be detected for 12(75%) of 16 patients' nasopharyngeal specimens in the combination group after seven days, compared with 6 (35%) of 17 in the monotherapy group (p < 0·05). After 14 days, 15 (94%) of 16 and 9 (52·9%) of 17, respectively, SARS-CoV-2 could not be detected (p < 0·05). The chest CT scans were improving for 11(69%) of 16 patients in the combination group after seven days, compared with 5(29%) of 17 in the monotherapy group (p < 0·05).
Conclusion: In patients with COVID-19, the apparent favorable clinical response with arbidol and LPV/r supports further LPV/r only.
Keywords: Antiviral intervention; Arbidol; Combination therapy; Corona Virus Disease 2019; Lopinavir/ritonavir.
Copyright © 2020 The British Infection Association. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures
References
-
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020 2020-02-19. [Epub ahead of print] - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

