Single-Photon Emission Computed Tomography Imaging Using Formyl Peptide Receptor 1 Ligand Can Diagnose Aortic Aneurysms in a Mouse Model
- PMID: 32172010
- PMCID: PMC7540921
- DOI: 10.1016/j.jss.2020.01.028
Single-Photon Emission Computed Tomography Imaging Using Formyl Peptide Receptor 1 Ligand Can Diagnose Aortic Aneurysms in a Mouse Model
Abstract
Background: Our previous studies showed that neutrophil infiltration and activation plays an important role in the pathogenesis of abdominal aortic aneurysms (AAA). However, there is a lack of noninvasive, inflammatory cell-specific molecular imaging methods to provide early diagnosis of AAA formation. Formyl peptide receptor 1 (FPR1) is rapidly upregulated on neutrophils during inflammation. Therefore, it is hypothesized that the use of cinnamoyl-F-(D)L-F-(D)L-F-K (cFLFLF), a PEGylated peptide ligand that binds FPR1 on activated neutrophils, would permit accurate and noninvasive diagnosis of AAA via single-photon emission computed tomography (SPECT) imaging.
Materials and methods: Male C57BL/6 (wild-type) mice were treated with topical elastase (0.4 U/mL type 1 porcine pancreatic elastase) or heat-inactivated elastase (control), and aortic diameter was measured by video micrometry. Comparative histology was performed on Day 14 to assess neutrophil infiltration in aortic tissue. We performed near-infrared fluorescence imaging using c-FLFLF-Cy7 probe on Days 7 and 14 postelastase treatment and measured fluorescence intensity ex vivo in excised aortic tissue. A separate group of animals were injected with 99mTc-c-FLFLF 2 h before SPECT imaging on Day 14 using a SPECT/computed tomography/positron emission tomography trimodal scanner. Coexpression of neutrophils with c-FLFLF was also performed on aortic tissue by immunostaining on Day 14.
Results: Aortic diameter was significantly increased in the elastase group compared with controls on Days 7 and 14. Simultaneously, a marked increase in neutrophil infiltration and elastin degradation as well as decrease in smooth muscle integrity were observed in aortic tissue after elastase treatment compared with controls. Moreover, a significant increase in fluorescence intensity of c-FLFLF-Cy7 imaging probe was also observed in elastase-treated mice on Day 7 (approximately twofold increase) and Day 14 (approximately 2.5-fold increase) compared with respective controls. SPECT imaging demonstrated a multifold increase in signal intensity for 99mTc-cFLFLF radiolabel probe in mice with AAA compared with controls on Day 14. Immunostaining of aortic tissue with c-FLFLF-Cy5 demonstrated a marked increase in coexpression with neutrophils in AAA compared with controls.
Conclusions: cFLFLF, a novel FPR1 ligand, enables quantifiable, noninvasive diagnosis and progression of AAAs. Clinical application of this inflammatory, cell-specific molecular probe using SPECT imaging may permit early diagnosis of AAA formation, enabling targeted therapeutic interventions and preventing impending aortic rupture.
Keywords: Abdominal aortic aneurysms; Aortic inflammation; Formyl peptide receptor; SPECT scans; Vascular imaging.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



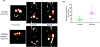

Similar articles
-
SPECT imaging of lung ischemia-reperfusion injury using [99mTc]cFLFLF for molecular targeting of formyl peptide receptor 1.Am J Physiol Lung Cell Mol Physiol. 2020 Feb 1;318(2):L304-L313. doi: 10.1152/ajplung.00220.2018. Epub 2019 Dec 4. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31800262 Free PMC article.
-
A Novel Modality for Functional Imaging in Acute Intervertebral Disk Herniation via Tracking Leukocyte Infiltration.Mol Imaging Biol. 2017 Oct;19(5):703-713. doi: 10.1007/s11307-016-1038-6. Mol Imaging Biol. 2017. PMID: 28050750 Free PMC article.
-
Deficiency of FAM3D (Family With Sequence Similarity 3, Member D), A Novel Chemokine, Attenuates Neutrophil Recruitment and Ameliorates Abdominal Aortic Aneurysm Development.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1616-1631. doi: 10.1161/ATVBAHA.118.311289. Epub 2018 May 31. Arterioscler Thromb Vasc Biol. 2018. PMID: 29853563 Free PMC article.
-
The potential impacts of formyl peptide receptor 1 in inflammatory diseases.Front Biosci (Elite Ed). 2016 Jun 1;8(3):436-49. doi: 10.2741/E778. Front Biosci (Elite Ed). 2016. PMID: 27100350 Review.
-
Tuberculosis: Role of Nuclear Medicine and Molecular Imaging With Potential Impact of Neutrophil-Specific Tracers.Front Med (Lausanne). 2021 Dec 10;8:758636. doi: 10.3389/fmed.2021.758636. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34957144 Free PMC article. Review.
Cited by
-
Resolution of inflammation via RvD1/FPR2 signaling mitigates Nox2 activation and ferroptosis of macrophages in experimental abdominal aortic aneurysms.FASEB J. 2022 Nov;36(11):e22579. doi: 10.1096/fj.202201114R. FASEB J. 2022. PMID: 36183323 Free PMC article.
-
B Cell-Activating Factor Antagonism Attenuates the Growth of Experimental Abdominal Aortic Aneurysm.Am J Pathol. 2021 Dec;191(12):2231-2244. doi: 10.1016/j.ajpath.2021.08.012. Epub 2021 Sep 9. Am J Pathol. 2021. PMID: 34509440 Free PMC article.
-
Retention of 64Cu-FLFLF, a Formyl Peptide Receptor 1-Specific PET Probe, Correlates with Macrophage and Neutrophil Abundance in Lung Granulomas from Cynomolgus Macaques.ACS Infect Dis. 2021 Aug 13;7(8):2264-2276. doi: 10.1021/acsinfecdis.0c00826. Epub 2021 Jul 13. ACS Infect Dis. 2021. PMID: 34255474 Free PMC article.
-
Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression.Life (Basel). 2021 Mar 15;11(3):243. doi: 10.3390/life11030243. Life (Basel). 2021. PMID: 33804219 Free PMC article. Review.
-
A fluorescent photoaffinity probe for formyl peptide receptor 1 labelling in living cells.RSC Chem Biol. 2023 Jan 13;4(3):216-222. doi: 10.1039/d2cb00199c. eCollection 2023 Mar 8. RSC Chem Biol. 2023. PMID: 36908701 Free PMC article.
References
-
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL and Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–30. - PubMed
-
- Lederle FA, Johnson GR, Wilson SE, Littooy FN, Krupski WC, Bandyk D, Acher CW, Chute EP, Hye RJ, Gordon IL, Freischlag J, Averbook AW and Makaroun MS. Yield of repeated screening for abdominal aortic aneurysm after a 4-year interval. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1117–21. - PubMed
-
- Cosford PA and Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007:CD002945. - PubMed
-
- Brewster DC, Cronenwett JL, Hallett JW Jr., Johnston KW, Krupski WC, Matsumura JS, Joint Council of the American Association for Vascular S and Society for Vascular S. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

