Drug-Induced Liver Injury: Clinical and Etiologic Features at a Large Tertiary Teaching Hospital in China
- PMID: 32172275
- PMCID: PMC7094059
- DOI: 10.12659/MSM.919435
Drug-Induced Liver Injury: Clinical and Etiologic Features at a Large Tertiary Teaching Hospital in China
Abstract
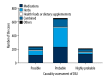
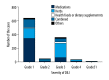
BACKGROUND Since the epidemiological profile of drug-induced liver injury (DILI) in China, especially the western of China, it has rarely been studied. The aim of this study was to analyze the characteristics of DILI patients in a large tertiary teaching hospital at Chongqing, a municipality in western China. MATERIAL AND METHODS The medical records of hospitalized patients which diagnosed with DILI between January 2011 and December 2016 were searched retrospectively, and demographic, clinical data, and laboratory data were retrieved for analysis. RESULTS A total of 1811 patients had been diagnosed with DILI, accounting for 0.248% of the total admissions during the same period. Among the 1096 patients included in our analysis, DILI was caused by "medications" in 462 cases (42.15%), "herbs" in 391 cases (35.68%), and combined medications in 189 cases (17.24%). The profiles for each etiology were distinctive for age, sex, clinical features, laboratory features, and types and severity of DILI. CONCLUSIONS Our study provides a systematic etiological profile of DILI in Chinese patients, which can represent references for prevention, diagnosis and treatment, supporting and promoting efforts to ease the burden of this liver disease in China.
Conflict of interest statement
None.
Figures


References
-
- Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102(3):558–62. - PubMed
-
- Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876–8. - PubMed
-
- Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34(02):134–44. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

