Plasma cells, plasmablasts, and AID+/CD30+ B lymphoblasts inside and outside germinal centres: details of the basal light zone and the outer zone in human palatine tonsils
- PMID: 32172287
- PMCID: PMC7343761
- DOI: 10.1007/s00418-020-01861-1
Plasma cells, plasmablasts, and AID+/CD30+ B lymphoblasts inside and outside germinal centres: details of the basal light zone and the outer zone in human palatine tonsils
Abstract
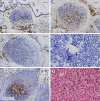
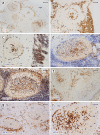
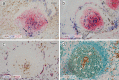
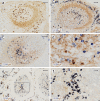
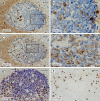
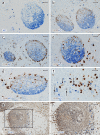
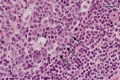
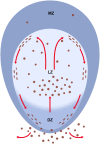
Plasma cells (PCs) in human palatine tonsils are predominantly located in the germinal centres (GCs), in the subepithelial space and near the deep connective tissue septa surrounding each crypt. We analysed the location, phenotype, and proliferation of GC PCs by immunohistology comparing them to PCs in the other two locations. Most PCs in GCs were strongly positive for CD38, CD138, CD27, IRF4, and intracellular (ic) IgG. They often accumulated in the basal light zone, but could also be found scattered in the entire light zone. In addition, rows of PCs occurred at the surface of the GC bordering the mantle zone, i.e., in the outer zone, and at the surface of the dark zone. The latter cells were often continuous with PCs in the extrafollicular area. The vast majority of GC PCs were negative for Ki-67. Only a few Ki-67+ plasmablasts, predominantly icIgG+ or icIgM+, were found inside GCs. In certain GCs PCs accumulated around capillaries and the adjacent perikarya of follicular dendritic cells (FDCs). Newly formed PCs might migrate from the basal to the superficial part of the light zone and then back to the dark zone surface to leave the GC. This guarantees an even distribution of secreted Ig for exchange with immune complexes on FDCs. The surface of the dark zone may also be an exit site for Ki-67+CD30+ B lymphoblasts, which seed perifollicular and extrafollicular sites. We speculate that these cells tend to downmodulate CD20 and activation-induced deaminase and further up-regulate CD30 when developing into pre-plasmablasts.
Keywords: B lymphoblasts; Germinal centres; Palatine tonsils; Plasma cells; Plasmablasts.
Conflict of interest statement
The authors are not aware of any conflict of interest.
Figures



Similar articles
-
Committed Human CD23-Negative Light-Zone Germinal Center B Cells Delineate Transcriptional Program Supporting Plasma Cell Differentiation.Front Immunol. 2021 Dec 2;12:744573. doi: 10.3389/fimmu.2021.744573. eCollection 2021. Front Immunol. 2021. PMID: 34925321 Free PMC article.
-
Human CD27+ memory B cells colonize a superficial follicular zone in the palatine tonsils with similarities to the spleen. A multicolor immunofluorescence study of lymphoid tissue.PLoS One. 2020 Mar 18;15(3):e0229778. doi: 10.1371/journal.pone.0229778. eCollection 2020. PLoS One. 2020. PMID: 32187186 Free PMC article.
-
Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells.Blood. 2006 May 15;107(10):3967-75. doi: 10.1182/blood-2005-10-4170. Epub 2006 Jan 26. Blood. 2006. PMID: 16439679
-
Germinal center B-cells.Autoimmunity. 2012 Aug;45(5):333-47. doi: 10.3109/08916934.2012.665524. Epub 2012 Apr 2. Autoimmunity. 2012. PMID: 22390182 Review.
-
IL-17 superfamily cytokines modulate normal germinal center B cell migration.J Leukoc Biol. 2016 Nov;100(5):913-918. doi: 10.1189/jlb.1VMR0216-096RR. Epub 2016 Aug 26. J Leukoc Biol. 2016. PMID: 27566830 Review.
Cited by
-
Clonal composition and differentiation stage of human CD30+ B cells in reactive lymph nodes.Front Immunol. 2023 Jul 25;14:1208610. doi: 10.3389/fimmu.2023.1208610. eCollection 2023. Front Immunol. 2023. PMID: 37559724 Free PMC article.
-
CNTools: A computational toolbox for cellular neighborhood analysis from multiplexed images.PLoS Comput Biol. 2024 Aug 28;20(8):e1012344. doi: 10.1371/journal.pcbi.1012344. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39196899 Free PMC article.
-
An atlas of cells in the human tonsil.Immunity. 2024 Feb 13;57(2):379-399.e18. doi: 10.1016/j.immuni.2024.01.006. Epub 2024 Jan 31. Immunity. 2024. PMID: 38301653 Free PMC article.
-
Multiscale Modeling of Germinal Center Recapitulates the Temporal Transition From Memory B Cells to Plasma Cells Differentiation as Regulated by Antigen Affinity-Based Tfh Cell Help.Front Immunol. 2021 Feb 5;11:620716. doi: 10.3389/fimmu.2020.620716. eCollection 2020. Front Immunol. 2021. PMID: 33613551 Free PMC article.
-
Optimizing assessment of CD30 expression in Hodgkin lymphoma by controlling for low expression.Histol Histopathol. 2024 Mar;39(3):319-331. doi: 10.14670/HH-18-644. Epub 2023 Jun 21. Histol Histopathol. 2024. PMID: 37377225 Free PMC article.
References
-
- Brachtel EF, Washiyama M, Johnson GD, Tenner-Racz K, Racz P, MacLennan IC. Differences in the germinal centres of palatine tonsils and lymph nodes. Scand J Immunol. 1996;43:239–247. - PubMed
-
- Cattoretti G. MYC expression and distribution in normal mature lymphoid cells. J Pathol. 2013;229:430–440. - PubMed
-
- Cattoretti G, Büttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–3975. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

