Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling
- PMID: 32172302
- PMCID: PMC7492447
- DOI: 10.1007/s00018-020-03494-y
Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling
Abstract
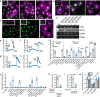
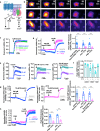
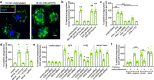
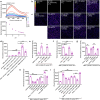
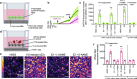
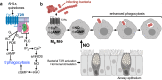
Bitter taste receptors (T2Rs) are GPCRs involved in detection of bitter compounds by type 2 taste cells of the tongue, but are also expressed in other tissues throughout the body, including the airways, gastrointestinal tract, and brain. These T2Rs can be activated by several bacterial products and regulate innate immune responses in several cell types. Expression of T2Rs has been demonstrated in immune cells like neutrophils; however, the molecular details of their signaling are unknown. We examined mechanisms of T2R signaling in primary human monocyte-derived unprimed (M0) macrophages (M[Formula: see text]s) using live cell imaging techniques. Known bitter compounds and bacterial T2R agonists activated low-level calcium signals through a pertussis toxin (PTX)-sensitive, phospholipase C-dependent, and inositol trisphosphate receptor-dependent calcium release pathway. These calcium signals activated low-level nitric oxide (NO) production via endothelial and neuronal NO synthase (NOS) isoforms. NO production increased cellular cGMP and enhanced acute phagocytosis ~ threefold over 30-60 min via protein kinase G. In parallel with calcium elevation, T2R activation lowered cAMP, also through a PTX-sensitive pathway. The cAMP decrease also contributed to enhanced phagocytosis. Moreover, a co-culture model with airway epithelial cells demonstrated that NO produced by epithelial cells can also acutely enhance M[Formula: see text] phagocytosis. Together, these data define M[Formula: see text] T2R signal transduction and support an immune recognition role for T2Rs in M[Formula: see text] cell physiology.
Keywords: Airway epithelium; G-protein-coupled receptors; Innate immunity; Live cell imaging; Quorum sensing.
Figures


Similar articles
-
Loss of CFTR function is associated with reduced bitter taste receptor-stimulated nitric oxide innate immune responses in nasal epithelial cells and macrophages.Front Immunol. 2023 Jan 18;14:1096242. doi: 10.3389/fimmu.2023.1096242. eCollection 2023. Front Immunol. 2023. PMID: 36742335 Free PMC article.
-
Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling.J Biol Chem. 2018 Jun 22;293(25):9824-9840. doi: 10.1074/jbc.RA117.001005. Epub 2018 May 10. J Biol Chem. 2018. PMID: 29748385 Free PMC article.
-
HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages.Cells. 2022 Apr 27;11(9):1478. doi: 10.3390/cells11091478. Cells. 2022. PMID: 35563784 Free PMC article.
-
A deadly taste: linking bitter taste receptors and apoptosis.Apoptosis. 2025 Apr;30(3-4):674-692. doi: 10.1007/s10495-025-02091-3. Epub 2025 Feb 20. Apoptosis. 2025. PMID: 39979526 Free PMC article. Review.
-
The pharmacology of bitter taste receptors and their role in human airways.Pharmacol Ther. 2015 Nov;155:11-21. doi: 10.1016/j.pharmthera.2015.08.001. Epub 2015 Aug 10. Pharmacol Ther. 2015. PMID: 26272040 Review.
Cited by
-
Bitter Taste Receptors (T2Rs) are Sentinels that Coordinate Metabolic and Immunological Defense Responses.Curr Opin Physiol. 2021 Apr;20:70-76. doi: 10.1016/j.cophys.2021.01.006. Epub 2021 Jan 12. Curr Opin Physiol. 2021. PMID: 33738371 Free PMC article.
-
Bitter Taste Receptor as a Therapeutic Target in Orthopaedic Disorders.Drug Des Devel Ther. 2021 Feb 26;15:895-903. doi: 10.2147/DDDT.S289614. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33679130 Free PMC article. Review.
-
TAS2R16 Activation Suppresses LPS-Induced Cytokine Expression in Human Gingival Fibroblasts.Front Immunol. 2021 Dec 15;12:726546. doi: 10.3389/fimmu.2021.726546. eCollection 2021. Front Immunol. 2021. PMID: 34975834 Free PMC article.
-
Loss of CFTR function is associated with reduced bitter taste receptor-stimulated nitric oxide innate immune responses in nasal epithelial cells and macrophages.Front Immunol. 2023 Jan 18;14:1096242. doi: 10.3389/fimmu.2023.1096242. eCollection 2023. Front Immunol. 2023. PMID: 36742335 Free PMC article.
-
The Multiple Faces of Nitric Oxide in Chronic Granulomatous Disease: A Comprehensive Update.Biomedicines. 2022 Oct 14;10(10):2570. doi: 10.3390/biomedicines10102570. Biomedicines. 2022. PMID: 36289832 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

