Growth-Promoting Treatment Screening for Corticospinal Neurons in Mouse and Man
- PMID: 32172457
- PMCID: PMC7497511
- DOI: 10.1007/s10571-020-00820-7
Growth-Promoting Treatment Screening for Corticospinal Neurons in Mouse and Man
Abstract
Neurons of the central nervous system (CNS) that project long axons into the spinal cord have a poor axon regenerative capacity compared to neurons of the peripheral nervous system. The corticospinal tract (CST) is particularly notorious for its poor regeneration. Because of this, traumatic spinal cord injury (SCI) is a devastating condition that remains as yet uncured. Based on our recent observations that direct neuronal interleukin-4 (IL-4) signaling leads to repair of axonal swellings and beneficial effects in neuroinflammation, we hypothesized that IL-4 acts directly on the CST. Here, we developed a tissue culture model for CST regeneration and found that IL-4 promoted new growth cone formation after axon transection. Most importantly, IL-4 directly increased the regenerative capacity of both murine and human CST axons, which corroborates its regenerative effects in CNS damage. Overall, these findings serve as proof-of-concept that our CST regeneration model is suitable for fast screening of new treatments for SCI.
Keywords: Corticospinal tract; Growth-promoting treatment; Interleukin-4; Regeneration; Spinal cord injury.
Conflict of interest statement
Authors declare no relevant competing interests.
Figures

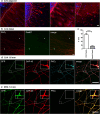

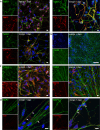

Similar articles
-
Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury.J Neurosci. 2013 Sep 25;33(39):15350-61. doi: 10.1523/JNEUROSCI.2510-13.2013. J Neurosci. 2013. PMID: 24068802 Free PMC article.
-
Pten Deletion Promotes Regrowth of Corticospinal Tract Axons 1 Year after Spinal Cord Injury.J Neurosci. 2015 Jul 1;35(26):9754-63. doi: 10.1523/JNEUROSCI.3637-14.2015. J Neurosci. 2015. PMID: 26134657 Free PMC article.
-
Modulation of Both Intrinsic and Extrinsic Factors Additively Promotes Rewiring of Corticospinal Circuits after Spinal Cord Injury.J Neurosci. 2021 Dec 15;41(50):10247-10260. doi: 10.1523/JNEUROSCI.2649-20.2021. Epub 2021 Nov 10. J Neurosci. 2021. PMID: 34759029 Free PMC article.
-
Intrinsic regulation of axon regeneration after spinal cord injury: Recent advances and remaining challenges.Exp Neurol. 2022 Nov;357:114198. doi: 10.1016/j.expneurol.2022.114198. Epub 2022 Aug 6. Exp Neurol. 2022. PMID: 35944658 Review.
-
Spinal cord regeneration.Cell Transplant. 2014;23(4-5):573-611. doi: 10.3727/096368914X678427. Cell Transplant. 2014. PMID: 24816452 Review.
Cited by
-
Interleukin-4 receptor signaling modulates neuronal network activity.J Exp Med. 2022 Jun 6;219(6):e20211887. doi: 10.1084/jem.20211887. Epub 2022 May 19. J Exp Med. 2022. PMID: 35587822 Free PMC article.
References
-
- Abu-Rub M, McMahon S, Zeugolis DI, Windebank A, Pandit A (2010) Spinal cord injury in vitro: modelling axon growth inhibition. Drug Discov Today 15:436–443. 10.1016/j.drudis.2010.03.008 - PubMed
-
- Bagnard D, Chounlamountri N, Puschel AW, Bolz J (2001) Axonal surface molecules act in combination with semaphorin 3a during the establishment of corticothalamic projections. Cereb Cortex 11:278–285 - PubMed
-
- Bradke F, Fawcett JW, Spira ME (2012) Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13:183–193. 10.1038/nrn3176 - PubMed
-
- Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ (2008) The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci 28:14107–14120. 10.1523/jneurosci.2217-08.2008 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

