Endothelial Sphingolipid De Novo Synthesis Controls Blood Pressure by Regulating Signal Transduction and NO via Ceramide
- PMID: 32172624
- PMCID: PMC7145736
- DOI: 10.1161/HYPERTENSIONAHA.119.14507
Endothelial Sphingolipid De Novo Synthesis Controls Blood Pressure by Regulating Signal Transduction and NO via Ceramide
Abstract
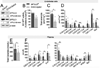
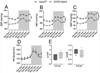
Ceramides are sphingolipids that modulate a variety of cellular processes via 2 major mechanisms: functioning as second messengers and regulating membrane biophysical properties, particularly lipid rafts, important signaling platforms. Altered sphingolipid levels have been implicated in many cardiovascular diseases, including hypertension, atherosclerosis, and diabetes mellitus-related conditions; however, molecular mechanisms by which ceramides impact endothelial functions remain poorly understood. In this regard, we generated mice defective of endothelial sphingolipid de novo biosynthesis by deleting the Sptlc2 (long chain subunit 2 of serine palmitoyltransferase)-the first enzyme of the pathway. Our study demonstrated that endothelial sphingolipid de novo production is necessary to regulate (1) signal transduction in response to NO agonists and, mainly via ceramides, (2) resting eNOS (endothelial NO synthase) phosphorylation, and (3) blood pressure homeostasis. Specifically, our findings suggest a prevailing role of C16:0-Cer in preserving vasodilation induced by tyrosine kinase and GPCRs (G-protein coupled receptors), except for Gq-coupled receptors, while C24:0- and C24:1-Cer control flow-induced vasodilation. Replenishing C16:0-Cer in vitro and in vivo reinstates endothelial cell signaling and vascular tone regulation. This study reveals an important role of locally produced ceramides, particularly C16:0-, C24:0-, and C24:1-Cer in vascular and blood pressure homeostasis, and establishes the endothelium as a key source of plasma ceramides. Clinically, specific plasma ceramides ratios are independent predictors of major cardiovascular events. Our data also suggest that plasma ceramides might be indicative of the diseased state of the endothelium.
Keywords: blood pressure; ceramides; endothelial; sphingolipids; vascular resistance.
Figures



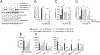
Similar articles
-
Sphingolipid Metabolism and Signaling in Endothelial Cell Functions.Adv Exp Med Biol. 2022;1372:87-117. doi: 10.1007/978-981-19-0394-6_8. Adv Exp Med Biol. 2022. PMID: 35503177 Review.
-
Apoptotic sphingolipid signaling by ceramides in lung endothelial cells.Am J Respir Cell Mol Biol. 2008 Jun;38(6):639-46. doi: 10.1165/rcmb.2007-0274OC. Epub 2008 Jan 10. Am J Respir Cell Mol Biol. 2008. PMID: 18192502 Free PMC article.
-
Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure.Nat Med. 2015 Sep;21(9):1028-1037. doi: 10.1038/nm.3934. Epub 2015 Aug 24. Nat Med. 2015. PMID: 26301690 Free PMC article.
-
Murine endothelial serine palmitoyltransferase 1 (SPTLC1) is required for vascular development and systemic sphingolipid homeostasis.Elife. 2022 Oct 5;11:e78861. doi: 10.7554/eLife.78861. Elife. 2022. PMID: 36197001 Free PMC article.
-
Sphingolipid synthetic pathways are major regulators of lipid homeostasis.Adv Exp Med Biol. 2011;721:139-48. doi: 10.1007/978-1-4614-0650-1_9. Adv Exp Med Biol. 2011. PMID: 21910087 Review.
Cited by
-
Emerging role of sphingolipids and extracellular vesicles in development and therapeutics of cardiovascular diseases.Int J Cardiol Heart Vasc. 2024 Jul 23;53:101469. doi: 10.1016/j.ijcha.2024.101469. eCollection 2024 Aug. Int J Cardiol Heart Vasc. 2024. PMID: 39139609 Free PMC article. Review.
-
Sphingolipids in the Heart: From Cradle to Grave.Front Endocrinol (Lausanne). 2020 Sep 15;11:652. doi: 10.3389/fendo.2020.00652. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042014 Free PMC article. Review.
-
Lysophosphatidic Acid-Mediated Inflammation at the Heart of Heart Failure.Curr Cardiol Rep. 2024 Mar;26(3):113-120. doi: 10.1007/s11886-024-02023-8. Epub 2024 Feb 10. Curr Cardiol Rep. 2024. PMID: 38340272 Review.
-
Sphingolipid Metabolism and Signaling in Endothelial Cell Functions.Adv Exp Med Biol. 2022;1372:87-117. doi: 10.1007/978-981-19-0394-6_8. Adv Exp Med Biol. 2022. PMID: 35503177 Review.
-
Essential Role of Endothelial Sphingolipid Biosynthesis in Cerebrovascular Homeostasis.Circ Res. 2023 Oct 27;133(10):880-882. doi: 10.1161/CIRCRESAHA.123.323183. Epub 2023 Oct 4. Circ Res. 2023. PMID: 37791485 Free PMC article. No abstract available.
References
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

