SPOT-Disorder2: Improved Protein Intrinsic Disorder Prediction by Ensembled Deep Learning
- PMID: 32173600
- PMCID: PMC7212484
- DOI: 10.1016/j.gpb.2019.01.004
SPOT-Disorder2: Improved Protein Intrinsic Disorder Prediction by Ensembled Deep Learning
Abstract
Intrinsically disordered or unstructured proteins (or regions in proteins) have been found to be important in a wide range of biological functions and implicated in many diseases. Due to the high cost and low efficiency of experimental determination of intrinsic disorder and the exponential increase of unannotated protein sequences, developing complementary computational prediction methods has been an active area of research for several decades. Here, we employed an ensemble of deep Squeeze-and-Excitation residual inception and long short-term memory (LSTM) networks for predicting protein intrinsic disorder with input from evolutionary information and predicted one-dimensional structural properties. The method, called SPOT-Disorder2, offers substantial and consistent improvement not only over our previous technique based on LSTM networks alone, but also over other state-of-the-art techniques in three independent tests with different ratios of disordered to ordered amino acid residues, and for sequences with either rich or limited evolutionary information. More importantly, semi-disordered regions predicted in SPOT-Disorder2 are more accurate in identifying molecular recognition features (MoRFs) than methods directly designed for MoRFs prediction. SPOT-Disorder2 is available as a web server and as a standalone program at https://sparks-lab.org/server/spot-disorder2/.
Keywords: Deep learning; Intrinsic disorder; Machine learning; Molecular recognition feature; Protein structure.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
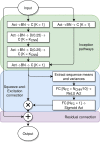


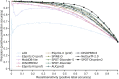
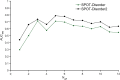
References
-
- Uversky V.N., Oldfield C.J., Dunker A.K. Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit. 2005;18:343–384. - PubMed
-
- Wright P.E., Dyson H.J. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. - PubMed
-
- Uversky V.N. Functions of short lifetime biological structures at large: the case of intrinsically disordered proteins. Brief Funct Genomics. 2018 Ely023. - PubMed
-
- Dyson H.J., Wright P.E. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

