Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and is Inhibited by Phosphatidylglycerol
- PMID: 32173651
- PMCID: PMC7174787
- DOI: 10.1124/mol.119.118166
Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and is Inhibited by Phosphatidylglycerol
Erratum in
-
Correction to "Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and Is Inhibited by Phosphatidylglycerol".Mol Pharmacol. 2020 May;97(5):354. doi: 10.1124/mol.119.118166err. Mol Pharmacol. 2020. PMID: 32299958 Free PMC article. No abstract available.
Abstract
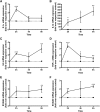
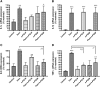
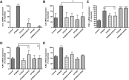
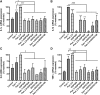
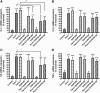
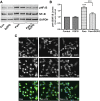
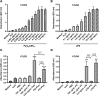
Skin serves not only as a protective barrier to microbial entry into the body but also as an immune organ. The outer layer, the epidermis, is composed predominantly of keratinocytes, which can be stimulated to produce proinflammatory mediators. Although some inflammation is useful to defend against infection, excessive or persistent inflammation can lead to the development of inflammatory skin diseases, such as psoriasis, a common skin disorder affecting approximately 2% of the US population. We have previously found that phosphatidylglycerol (PG) derived from soy can inhibit inflammation in a contact irritant ear edema mouse model. Here, we investigated the ability of soy PG to inhibit inflammatory mediator expression in response to activators of the pattern recognition receptors, toll-like receptor-2 (TLR2) and -4 (TLR4). We found that in epidermal keratinocytes, soy PG inhibited TLR2 and TLR4 activation and inflammatory mediator expression in response to a synthetic triacylated lipopeptide and lipopolysaccharide, respectively, as well as an endogenous danger-associated molecular pattern. However, at higher concentrations, soy PG alone enhanced the expression of some proinflammatory cytokines, suggesting a narrow therapeutic window for this lipid. Dioleoylphosphatidylglycerol (DOPG), but not dioleoylphosphatidylcholine, exerted a similar inhibitory effect, completely blocking keratinocyte inflammatory mediator expression induced by TLR2 and TLR4 activators as well as NFκB activation in a macrophage cell line (RAW264.7); however, DOPG was not itself proinflammatory even at high concentrations. Furthermore, DOPG had no effect on NFκB activation in response to a TLR7/8 agonist. Our results suggest that DOPG could be used to inhibit excessive skin inflammation. SIGNIFICANCE STATEMENT: Although inflammation is beneficial for clearing an infection, in some cases, the infection can be excessive and/or become chronic, thereby resulting in considerable tissue damage and pathological conditions. We show here that the phospholipid phosphatidylglycerol can inhibit the activation of toll-like receptors 2 and 4 of the innate immune system as well as the downstream inflammatory mediator expression in response to microbial component-mimicking agents in epidermal keratinocytes that form the physical barrier of the skin.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Phosphatidylglycerol Inhibits Toll-Like Receptor-Mediated Inflammation by Danger-Associated Molecular Patterns.J Invest Dermatol. 2019 Apr;139(4):868-877. doi: 10.1016/j.jid.2018.10.021. Epub 2018 Oct 31. J Invest Dermatol. 2019. PMID: 30391260 Free PMC article.
-
Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2.PLoS One. 2013 May 20;8(5):e63990. doi: 10.1371/journal.pone.0063990. Print 2013. PLoS One. 2013. PMID: 23700442 Free PMC article.
-
TLR2 and TLR4 signaling pathways are required for recombinant Brucella abortus BCSP31-induced cytokine production, functional upregulation of mouse macrophages, and the Th1 immune response in vivo and in vitro.Cell Mol Immunol. 2014 Sep;11(5):477-94. doi: 10.1038/cmi.2014.28. Epub 2014 Apr 28. Cell Mol Immunol. 2014. PMID: 24769793 Free PMC article.
-
Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes.Pharmaceutics. 2023 May 14;15(5):1497. doi: 10.3390/pharmaceutics15051497. Pharmaceutics. 2023. PMID: 37242739 Free PMC article. Review.
-
Innate Immune System Activation, Inflammation and Corneal Wound Healing.Int J Mol Sci. 2022 Nov 29;23(23):14933. doi: 10.3390/ijms232314933. Int J Mol Sci. 2022. PMID: 36499260 Free PMC article. Review.
Cited by
-
Aquaporin-3 in the epidermis: more than skin deep.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1144-C1153. doi: 10.1152/ajpcell.00075.2020. Epub 2020 Apr 8. Am J Physiol Cell Physiol. 2020. PMID: 32267715 Free PMC article. Review.
-
Advanced Glycation End Products and Activation of Toll-like Receptor-2 and -4 Induced Changes in Aquaporin-3 Expression in Mouse Keratinocytes.Int J Mol Sci. 2023 Jan 10;24(2):1376. doi: 10.3390/ijms24021376. Int J Mol Sci. 2023. PMID: 36674890 Free PMC article.
-
Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis.Int J Mol Sci. 2021 Aug 15;22(16):8749. doi: 10.3390/ijms22168749. Int J Mol Sci. 2021. PMID: 34445455 Free PMC article.
-
Therapeutic benefits of glycerol in dry eye disease.Front Med (Lausanne). 2025 Jan 15;11:1531670. doi: 10.3389/fmed.2024.1531670. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39882517 Free PMC article.
-
Increased Weight Gain and Insulin Resistance in HF-Fed PLTP Deficient Mice Is Related to Altered Inflammatory Response and Plasma Transport of Gut-Derived LPS.Int J Mol Sci. 2022 Oct 30;23(21):13226. doi: 10.3390/ijms232113226. Int J Mol Sci. 2022. PMID: 36362012 Free PMC article.
References
-
- Bollag WB, Ducote J, Harmon CS. (1993) Effects of the selective protein kinase C inhibitor, Ro 31-7549, on the proliferation of cultured mouse epidermal keratinocytes. J Invest Dermatol 100:240–246. - PubMed
-
- Bollag WB, Xie D, Zhong X, Zheng X. (2007) A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol 127:2823–2831. - PubMed
-
- Brotas AM, Cunha JM, Lago EH, Machado CC, Carneiro SC. (2012) Tumor necrosis factor-alpha and the cytokine network in psoriasis. An Bras Dermatol 87:673–681–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

