Combined refinements to somatic cell nuclear transfer methods improve porcine embryo development
- PMID: 32173679
- PMCID: PMC7297629
- DOI: 10.1262/jrd.2019-156
Combined refinements to somatic cell nuclear transfer methods improve porcine embryo development
Abstract
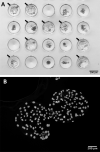
The discovery of how to utilize CRISPR (clustered, regularly interspaced, short, palindromic repeats)-Cas (CRISPR-associated) systems for genome modification has accelerated development of the field of genome editing, especially in large animals such as pigs. The low efficiency of somatic cell nuclear transfer (SCNT) is now becoming a major obstacle in the production of genome-edited animals via cell-mediated approaches and improving efficacy of this technique is crucial. In this study, we propose a few simple modifications to a zona-free SCNT protocol that are effective to produce numerous high-quality blastocysts. To refine the SCNT protocol we modified the following steps/factors: 1) culture medium for SCNT embryos, 2) chemical treatment to prevent precocious activation of the manipulated/reconstructed oocytes and 3) donor cell serum starvation treatment. Although changes in each of these steps only resulted in small improvements, the combination of all modifications altogether significantly enhanced developmental competence of SCNT embryos. Our modified method yielded approximately three times greater blastocyst formation rates. Moreover, resulting blastocysts had roughly twice as many cells as compared to blastocysts produced by the conventional SCNT method. With these significant in vitro improvements, our refined SCNT method is potentially suited for use in the production of genome edited pigs.
Keywords: Donor cells; Embryonic development competence; Pigs; Somatic cell nuclear transfer.
Figures
References
-
- Pennisi E. The CRISPR craze. Science 2013; 341: 833–836. - PubMed
-
- Barrangou R, May AP. Unraveling the potential of CRISPR-Cas9 for gene therapy. Expert Opin Biol Ther 2015; 15: 311–314. - PubMed
-
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014; 346: 1258096. - PubMed

