A morphometric analysis of the lungs of high-altitude ducks and geese
- PMID: 32173858
- PMCID: PMC7309286
- DOI: 10.1111/joa.13180
A morphometric analysis of the lungs of high-altitude ducks and geese
Abstract
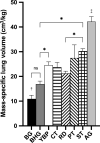
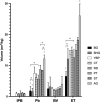
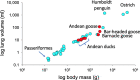
We examined the morphology of the lungs of five species of high-altitude resident ducks from Lake Titicaca in the Peruvian Andes (yellow-billed pintail [Anas georgica], cinnamon teal [Anas cyanoptera orinomus], puna teal [Anas puna], speckled teal [Anas flavirostris oxyptera], and ruddy duck [Oxyura jamaicensis ferruginea]) and compared them with those of the high-altitude migratory bar-headed goose (Anser indicus) and the low-altitude migratory barnacle goose (Branta leucopsis). We then determined the relationship between mass-specific lung volume, the volume densities of the component parts of the lung, and previously reported hypoxia-induced increases in pulmonary O2 extraction. We found that the mass-specific lung volumes and the mass-specific volume of the exchange tissue were larger in the lungs of high-altitude resident birds. The bar-headed goose had a mass-specific lung volume that fell between those of the low-altitude species and the high-altitude residents, but a mass-specific volume of exchange tissue that was not significantly different than that of the high-altitude residents. The data suggest that the mass-specific volume of the lung may increase with evolutionary time spent at altitude. We found an inverse relationship between the percentage increase in pulmonary O2 extraction and the percentage increase in ventilation across species that was independent of the volume density of the exchange tissue, at least for the resident Andean birds.
Keywords: altitude; birds; hypoxia; lungs; morphometry; pulmonary O2 extraction; pulmonary exchange tissue.
© 2020 Anatomical Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Respiratory mechanics and morphology of Tibetan and Andean high-altitude geese with divergent life histories.J Exp Biol. 2018 Jan 10;221(Pt 1):jeb170738. doi: 10.1242/jeb.170738. J Exp Biol. 2018. PMID: 29180602
-
Control of breathing and respiratory gas exchange in high-altitude ducks native to the Andes.J Exp Biol. 2019 Apr 1;222(Pt 7):jeb198622. doi: 10.1242/jeb.198622. J Exp Biol. 2019. PMID: 30846536
-
Divergent respiratory and cardiovascular responses to hypoxia in bar-headed geese and Andean birds.J Exp Biol. 2017 Nov 15;220(Pt 22):4186-4194. doi: 10.1242/jeb.168799. J Exp Biol. 2017. PMID: 29141880
-
Different strategies for convective O2 transport in high altitude birds: A graphical analysis.Comp Biochem Physiol A Mol Integr Physiol. 2021 Mar;253:110871. doi: 10.1016/j.cbpa.2020.110871. Epub 2020 Dec 13. Comp Biochem Physiol A Mol Integr Physiol. 2021. PMID: 33321176 Review.
-
High-altitude champions: birds that live and migrate at altitude.J Appl Physiol (1985). 2017 Oct 1;123(4):942-950. doi: 10.1152/japplphysiol.00110.2017. Epub 2017 Aug 24. J Appl Physiol (1985). 2017. PMID: 28839002 Free PMC article. Review.
Cited by
-
Gas exchange, oxygen transport and metabolism in high-altitude waterfowl.Philos Trans R Soc Lond B Biol Sci. 2025 Feb 27;380(1920):20230424. doi: 10.1098/rstb.2023.0424. Epub 2025 Feb 27. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40010396 Review.
-
The Andean Ibis (Theristicus branickii) in South America: potential distribution, presence in protected areas and anthropic threats.PeerJ. 2023 Dec 11;11:e16533. doi: 10.7717/peerj.16533. eCollection 2023. PeerJ. 2023. PMID: 38099301 Free PMC article.
-
High-altitude adaptation is accompanied by strong signatures of purifying selection in the mitochondrial genomes of three Andean waterfowl.PLoS One. 2024 Jan 3;19(1):e0294842. doi: 10.1371/journal.pone.0294842. eCollection 2024. PLoS One. 2024. PMID: 38170710 Free PMC article.
References
-
- Kooyman, G.L. and Ponganis, P.J. (2002) The physiological basis of diving to depth: birds and Mammals. Annual Review of Physiology, 60, 19–32. - PubMed
-
- Lague, S.L. , Chua, B. , Alza, L. , Scott, G.R. , Frappell, P.B., Zhong, Y et al (2017) Divergent respiratory and cardiovascular responses to hypoxia in bar‐headed geese and Andean birds. Journal of Experimental Biology, 220(22), 4186–4194. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

