Helicobacter pylori infection impairs chaperone-assisted maturation of Na-K-ATPase in gastric epithelium
- PMID: 32174134
- PMCID: PMC7272721
- DOI: 10.1152/ajpgi.00266.2019
Helicobacter pylori infection impairs chaperone-assisted maturation of Na-K-ATPase in gastric epithelium
Abstract
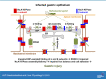
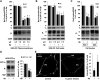
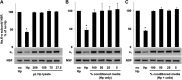
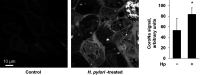
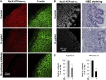
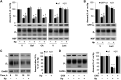
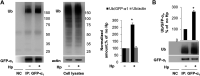
Helicobacter pylori infection always induces gastritis, which may progress to ulcer disease or cancer. The mechanisms underlying mucosal injury by the bacteria are incompletely understood. Here, we identify a novel pathway for H. pylori-induced gastric injury, the impairment of maturation of the essential transport enzyme and cell adhesion molecule, Na-K-ATPase. Na-K-ATPase comprises α- and β-subunits that assemble in the endoplasmic reticulum (ER) before trafficking to the plasma membrane. Attachment of H. pylori to gastric epithelial cells increased Na-K-ATPase ubiquitylation, decreased its surface and total levels, and impaired ion balance. H. pylori did not alter degradation of plasmalemma-resident Na-K-ATPase subunits or their mRNA levels. Infection decreased association of α- and β-subunits with ER chaperone BiP and impaired assembly of α/β-heterodimers, as was revealed by quantitative mass spectrometry and immunoblotting of immunoprecipitated complexes. The total level of BiP was not altered, and the decrease in interaction with BiP was not observed for other BiP client proteins. The H. pylori-induced decrease in Na-K-ATPase was prevented by BiP overexpression, stopping protein synthesis, or inhibiting proteasomal, but not lysosomal, protein degradation. The results indicate that H. pylori impairs chaperone-assisted maturation of newly made Na-K-ATPase subunits in the ER independently of a generalized ER stress and induces their ubiquitylation and proteasomal degradation. The decrease in Na-K-ATPase levels is also seen in vivo in the stomachs of gerbils and chronically infected children. Further understanding of H. pylori-induced Na-K-ATPase degradation will provide insights for protection against advanced disease.NEW & NOTEWORTHY This work provides evidence that Helicobacter pylori decreases levels of Na-K-ATPase, a vital transport enzyme, in gastric epithelia, both in acutely infected cultured cells and in chronically infected patients and animals. The bacteria interfere with BiP-assisted folding of newly-made Na-K-ATPase subunits in the endoplasmic reticulum, accelerating their ubiquitylation and proteasomal degradation and decreasing efficiency of the assembly of native enzyme. Decreased Na-K-ATPase expression contributes to H. pylori-induced gastric injury.
Keywords: Helicobacter pylori; Na-K-ATPase; endoplasmic reticulum; gastric epithelium; protein maturation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures


Similar articles
-
Helicobacter pylori-Induced Decrease in Membrane Expression of Na,K-ATPase Leads to Gastric Injury.Biomolecules. 2024 Jun 28;14(7):772. doi: 10.3390/biom14070772. Biomolecules. 2024. PMID: 39062486 Free PMC article.
-
Degradation and endoplasmic reticulum retention of unassembled alpha- and beta-subunits of Na,K-ATPase correlate with interaction of BiP.J Biol Chem. 1996 Aug 23;271(34):20895-902. doi: 10.1074/jbc.271.34.20895. J Biol Chem. 1996. PMID: 8702846
-
Diverse pathways for maturation of the Na,K-ATPase β1 and β2 subunits in the endoplasmic reticulum of Madin-Darby canine kidney cells.J Biol Chem. 2010 Dec 10;285(50):39289-302. doi: 10.1074/jbc.M110.172858. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937802 Free PMC article.
-
Polarized membrane distribution of potassium-dependent ion pumps in epithelial cells: different roles of the N-glycans of their beta subunits.Cell Biochem Biophys. 2007;47(3):376-91. doi: 10.1007/s12013-007-0033-6. Cell Biochem Biophys. 2007. PMID: 17652782 Review.
-
Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis.World J Gastroenterol. 2015 Dec 7;21(45):12742-56. doi: 10.3748/wjg.v21.i45.12742. World J Gastroenterol. 2015. PMID: 26668499 Free PMC article. Review.
Cited by
-
Effect of Essential Oils on the Oxyntopeptic Cells and Somatostatin and Ghrelin Immunoreactive Cells in the European Sea Bass (Dicentrarchus labrax) Gastric Mucosa.Animals (Basel). 2021 Nov 29;11(12):3401. doi: 10.3390/ani11123401. Animals (Basel). 2021. PMID: 34944178 Free PMC article.
-
Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review).J Clin Med. 2020 Oct 12;9(10):3253. doi: 10.3390/jcm9103253. J Clin Med. 2020. PMID: 33053671 Free PMC article. Review.
-
Proline metabolism is essential for alkaline adaptation of Nile tilapia (Oreochromis niloticus).J Anim Sci Biotechnol. 2024 Oct 14;15(1):142. doi: 10.1186/s40104-024-01100-w. J Anim Sci Biotechnol. 2024. PMID: 39397002 Free PMC article.
-
Molecular mechanisms of Na,K-ATPase dysregulation driving alveolar epithelial barrier failure in severe COVID-19.Am J Physiol Lung Cell Mol Physiol. 2021 Jun 1;320(6):L1186-L1193. doi: 10.1152/ajplung.00056.2021. Epub 2021 Mar 9. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33689516 Free PMC article.
-
Chemical components and protective effects of Atractylodes japonica Koidz. ex Kitam against acetic acid-induced gastric ulcer in rats.World J Gastroenterol. 2023 Nov 21;29(43):5848-5864. doi: 10.3748/wjg.v29.i43.5848. World J Gastroenterol. 2023. PMID: 38074916 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

